当前位置:
X-MOL 学术
›
Resour. Geol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organic matters and acid‐sulfate alteration in Itomuka mercury mine, Hokkaido, Japan: Implications for the transportation and deposition mechanisms of Hg
Resource Geology ( IF 1.1 ) Pub Date : 2019-12-17 , DOI: 10.1111/rge.12225 Takuya Echigo 1 , Mitsuyoshi Kimata 2 , Masahiro Shimizu 2
Resource Geology ( IF 1.1 ) Pub Date : 2019-12-17 , DOI: 10.1111/rge.12225 Takuya Echigo 1 , Mitsuyoshi Kimata 2 , Masahiro Shimizu 2
Affiliation
|
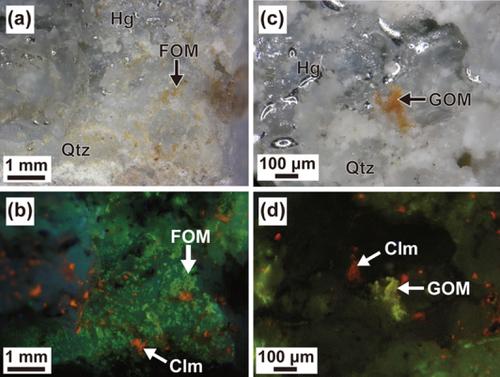
In order to examine the transportation and deposition mechanisms of Hg, we investigated the ore and hydrothermal alteration minerals and solid organic matters from Itomuka mercury mine located in the eastern part of central Hokkaido. In addition to the ore minerals, native mercury and cinnabar, quartz, marcasite, alunite, kaolinite, and minor amounts of pyrite and smectite were identified in the Hg ore by powder X‐ray diffraction (XRD) analysis. This mineral assemblage of acid sulfate alteration was likely developed under the conditions of low temperature (≤100°C) and low pH (≤2) in the steam‐heated environment. The H2SO4 was produced above the water table by the oxidation of H2S separated from deep, near‐neutral fluids by boiling. The dominance of native mercury over cinnabar in Hg ore indicates that the greater part of mineralized Hg was transported as Hg0 in aqueous solution and vapor with low sulfur fugacity. The solid organic matters found in the Hg ore were analyzed with SEM‐EDS, micro‐XRD, and micro‐Fourier transform infrared (FTIR) spectroscopy, and these results suggest that the organic matters contributed to keeping the low fO2 of the Hg‐bearing fluid and transportation of Hg as Hg0 in S‐poor condition. Because the solubility of Hg in acidic fluid is low, neutral to alkaline fluid seems to have leached Hg from the basement sedimentary rocks of Hidaka Group which also supplied the organic matters to the fluid. The oxidation and cooling of Hg‐bearing solution and vapor triggered the deposition of liquid Hg as a primary phase.
中文翻译:

日本北海道板冢汞矿中的有机物和酸硫酸盐蚀变:对汞的迁移和沉积机理的启示
为了研究汞的迁移和沉积机理,我们调查了北海道中部东部板岩汞矿的矿石,热液蚀变矿物和固体有机物。通过粉末X射线衍射(XRD)分析,在汞矿石中还鉴定出了矿石矿物,天然汞和朱砂,石英,镁铁矿,铝矾石,高岭石以及少量的黄铁矿和绿土。这种酸性硫酸盐蚀变的矿物组合很可能是在蒸汽加热的环境中在低温(≤100°C)和低pH(≤2)的条件下形成的。为H 2 SO 4中的H氧化地下水位上述制作2S通过沸腾与深的,接近中性的流体分离。汞矿石中天然汞占朱砂的优势表明,矿化汞的大部分以Hg 0的形式在低硫逸度的水溶液和蒸气中运输。使用SEM-EDS,micro-XRD和micro-Fourier变换红外(FTIR)光谱分析了汞矿石中发现的固体有机物,这些结果表明有机物有助于保持汞的低f O 2。含汞流体和汞的运输形式为汞0处于低劣状态。由于Hg在酸性流体中的溶解度较低,因此中性至碱性流体似乎已从Hidaka Group的基底沉积岩中浸出了Hg,Hdaka Group的地下沉积岩也向流体中提供了有机物。含汞溶液和蒸气的氧化和冷却触发了液态汞作为主要相的沉积。
更新日期:2019-12-17
中文翻译:

日本北海道板冢汞矿中的有机物和酸硫酸盐蚀变:对汞的迁移和沉积机理的启示
为了研究汞的迁移和沉积机理,我们调查了北海道中部东部板岩汞矿的矿石,热液蚀变矿物和固体有机物。通过粉末X射线衍射(XRD)分析,在汞矿石中还鉴定出了矿石矿物,天然汞和朱砂,石英,镁铁矿,铝矾石,高岭石以及少量的黄铁矿和绿土。这种酸性硫酸盐蚀变的矿物组合很可能是在蒸汽加热的环境中在低温(≤100°C)和低pH(≤2)的条件下形成的。为H 2 SO 4中的H氧化地下水位上述制作2S通过沸腾与深的,接近中性的流体分离。汞矿石中天然汞占朱砂的优势表明,矿化汞的大部分以Hg 0的形式在低硫逸度的水溶液和蒸气中运输。使用SEM-EDS,micro-XRD和micro-Fourier变换红外(FTIR)光谱分析了汞矿石中发现的固体有机物,这些结果表明有机物有助于保持汞的低f O 2。含汞流体和汞的运输形式为汞0处于低劣状态。由于Hg在酸性流体中的溶解度较低,因此中性至碱性流体似乎已从Hidaka Group的基底沉积岩中浸出了Hg,Hdaka Group的地下沉积岩也向流体中提供了有机物。含汞溶液和蒸气的氧化和冷却触发了液态汞作为主要相的沉积。









































 京公网安备 11010802027423号
京公网安备 11010802027423号