当前位置:
X-MOL 学术
›
J. Label. Comp. Radiopharm.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Selective synthesis of L-2-[18 F]fluoro-alpha-methylphenylalanine via copper mediated 18 F-fluorination of (mesityl)(aryl)iodonium salt
Journal of Labelled Compounds and Radiopharmaceuticals ( IF 0.9 ) Pub Date : 2020-04-28 , DOI: 10.1002/jlcr.3840 Aiko Yamaguchi 1, 2 , Hirofumi Hanaoka 1 , Tetsuya Higuchi 3 , Yoshito Tsushima 3, 4
Journal of Labelled Compounds and Radiopharmaceuticals ( IF 0.9 ) Pub Date : 2020-04-28 , DOI: 10.1002/jlcr.3840 Aiko Yamaguchi 1, 2 , Hirofumi Hanaoka 1 , Tetsuya Higuchi 3 , Yoshito Tsushima 3, 4
Affiliation
|
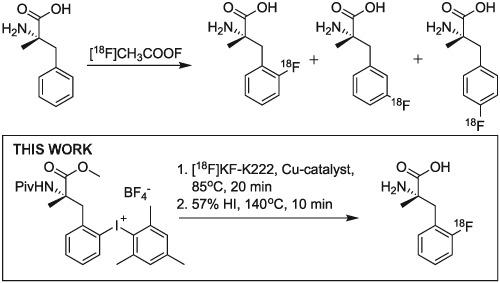
L-2-[18 F]fluoro-alpha-methylphenylalanine (2-[18 F]FAMP) is a promising amino acid tracer for positron emission tomography (PET) imaging, yet the low production yield of direct electrophilic radiofluorination with [18 F]F2 necessitates further optimization of the radiolabeling process. This paper describes a 2-step preparation method for L-2-[18 F]fluoro-alpha-methylphenylalanine (2-[18 F]FAMP) starting from [18 F]fluoride. The (Mesityl)(L-alpha-methylphenylalanine)-2-iodonium tetrafluoroborate precursors with various protecting groups were prepared. The copper-mediated 18 F-fluorination of the iodonium salt precursors successfully produced 2-[18 F]FAMP. The highest radio chemical conversion of 57.6% was noted with N-Piv-protected (mesityl)(aryl)iodonium salt in the presence of 5 equivalent of Cu (OTf)2 . Subsequent deprotection with 57% hydrogen iodide produced 2-[18 F]FAMP within 120 min in 21.4±11.7% overall radiochemical yield with >95% radiochemical purity and an enantiomeric excess >99%. The obtained 2-[18 F]FAMP showed comparable biodistribution profiles in normal mice with that of the carrier-added 2-[18 F]FAMP. These results indicate that usefulness of copper mediated 18 F-fluorination for the production of 2-[18 F]FAMP, which would facilitate clinical translation of the promising tumor specific amino acid tracer. Individual facilities could adopt either production method based on radioactivity demand and equipment availability.
中文翻译:

通过铜介导的(异三叉基)(芳基)碘鎓盐的 18 F-氟化选择性合成 L-2-[18 F]氟-α-甲基苯丙氨酸
L-2-[18 F]氟-α-甲基苯丙氨酸 (2-[18 F]FAMP) 是一种有前途的用于正电子发射断层扫描 (PET) 成像的氨基酸示踪剂,但 [18 F] 直接亲电放射性氟化的产率较低]F2 需要进一步优化放射性标记过程。本文描述了一种以[18 F]氟化物为原料的L-2-[18 F]氟-α-甲基苯丙氨酸(2-[18 F]FAMP)的两步制备方法。制备了具有各种保护基团的(异丙基)(L-α-甲基苯丙氨酸)-2-碘鎓四氟硼酸盐前体。碘鎓盐前体的铜介导的 18 F-氟化成功地产生了 2-[18 F]FAMP。在 5 当量的 Cu (OTf)2 存在下,N-Piv 保护的(异三叉基)(芳基)碘鎓盐的放射化学转化率最高为 57.6%。随后用 57% 碘化氢脱保护,在 120 分钟内产生 2-[18 F]FAMP,总放射化学产率为 21.4±11.7%,放射化学纯度为 >95%,对映体过量为 >99%。所获得的2-[18F]FAMP在正常小鼠中显示出与添加载体的2-[18F]FAMP相当的生物分布特征。这些结果表明铜介导的 18 F-氟化可用于生产 2-[18 F]FAMP,这将有助于有前途的肿瘤特异性氨基酸示踪剂的临床转化。各个设施可以根据放射性需求和设备可用性采用任一生产方法。
更新日期:2020-04-28
中文翻译:

通过铜介导的(异三叉基)(芳基)碘鎓盐的 18 F-氟化选择性合成 L-2-[18 F]氟-α-甲基苯丙氨酸
L-2-[18 F]氟-α-甲基苯丙氨酸 (2-[18 F]FAMP) 是一种有前途的用于正电子发射断层扫描 (PET) 成像的氨基酸示踪剂,但 [18 F] 直接亲电放射性氟化的产率较低]F2 需要进一步优化放射性标记过程。本文描述了一种以[18 F]氟化物为原料的L-2-[18 F]氟-α-甲基苯丙氨酸(2-[18 F]FAMP)的两步制备方法。制备了具有各种保护基团的(异丙基)(L-α-甲基苯丙氨酸)-2-碘鎓四氟硼酸盐前体。碘鎓盐前体的铜介导的 18 F-氟化成功地产生了 2-[18 F]FAMP。在 5 当量的 Cu (OTf)2 存在下,N-Piv 保护的(异三叉基)(芳基)碘鎓盐的放射化学转化率最高为 57.6%。随后用 57% 碘化氢脱保护,在 120 分钟内产生 2-[18 F]FAMP,总放射化学产率为 21.4±11.7%,放射化学纯度为 >95%,对映体过量为 >99%。所获得的2-[18F]FAMP在正常小鼠中显示出与添加载体的2-[18F]FAMP相当的生物分布特征。这些结果表明铜介导的 18 F-氟化可用于生产 2-[18 F]FAMP,这将有助于有前途的肿瘤特异性氨基酸示踪剂的临床转化。各个设施可以根据放射性需求和设备可用性采用任一生产方法。










































 京公网安备 11010802027423号
京公网安备 11010802027423号