当前位置:
X-MOL 学术
›
Mater. Corros.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of the addition of Na2SnO3 to NaCl electrolytes on Mg‐14Li‐3Al‐1Gd electrode electrochemical behavior
Materials and Corrosion ( IF 1.6 ) Pub Date : 2019-12-10 , DOI: 10.1002/maco.201911309 Guangyu Li 1 , Jinhao Su 1 , Xinyue Zhao 1 , Shixing Chen 1 , Ruizhi Wu 1 , Guixiang Wang 1
Materials and Corrosion ( IF 1.6 ) Pub Date : 2019-12-10 , DOI: 10.1002/maco.201911309 Guangyu Li 1 , Jinhao Su 1 , Xinyue Zhao 1 , Shixing Chen 1 , Ruizhi Wu 1 , Guixiang Wang 1
Affiliation
|
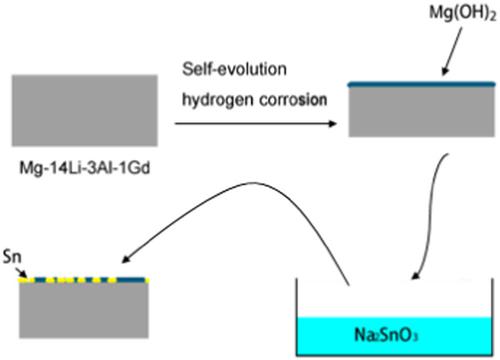
We report on the electrochemical performance of Mg‐14Li‐3Al‐1Gd electrodes prepared by the accumulation roll bonding technique in a 0.7 M NaCl solution. To explore the effects of adding different concentrations of Na2SnO3 to 0.7 M NaCl solutions, potentiodynamic polarization, potentiostatic oxidation, electrochemical impedance spectroscopy, and scanning electron microscopy were utilized. The results show that the addition of Na2SnO3 to a 0.7 M NaCl solution increases the corrosion potential of the Mg‐14Li‐3Al‐1Gd electrodes. Samples with 0.1 mM Na2SnO3 retained the highest discharging current density and lowest polarization resistance of all the specimens. Electrodes in an electrolyte solution mixed with 0.1 mM Na2SnO3 presented a larger active reaction area, deeper channels, and higher discharging currents than those with other additive concentrations. In conclusion, to improve the electrochemical behavior of Mg‐14Li‐3Al‐1Gd electrodes in a 0.7 M NaCl solution, the optimal concentration of Na2SnO3 is 0.1 mM.
中文翻译:

在NaCl电解液中添加Na2SnO3对Mg-14Li-3Al-1Gd电极电化学行为的影响
我们报告了在0.7 M NaCl溶液中通过累积辊压结合技术制备的Mg-14Li-3Al-1Gd电极的电化学性能。为了探索向0.7 M NaCl溶液中添加不同浓度的Na 2 SnO 3的影响,利用了电位动力学极化,恒电位氧化,电化学阻抗谱和扫描电子显微镜。结果表明,在0.7 M NaCl溶液中添加Na 2 SnO 3会增加Mg-14Li-3Al-1Gd电极的腐蚀电位。含0.1 mM Na 2 SnO 3的样品保留了所有样品的最高放电电流密度和最低极化电阻。与其他添加剂浓度相比,混合了0.1 mM Na 2 SnO 3的电解质溶液中的电极具有更大的活性反应面积,更深的通道和更高的放电电流。总之,为了改善Mg-14Li-3Al-1Gd电极在0.7 M NaCl溶液中的电化学行为,Na 2 SnO 3的最佳浓度为0.1 mM。
更新日期:2019-12-10
中文翻译:

在NaCl电解液中添加Na2SnO3对Mg-14Li-3Al-1Gd电极电化学行为的影响
我们报告了在0.7 M NaCl溶液中通过累积辊压结合技术制备的Mg-14Li-3Al-1Gd电极的电化学性能。为了探索向0.7 M NaCl溶液中添加不同浓度的Na 2 SnO 3的影响,利用了电位动力学极化,恒电位氧化,电化学阻抗谱和扫描电子显微镜。结果表明,在0.7 M NaCl溶液中添加Na 2 SnO 3会增加Mg-14Li-3Al-1Gd电极的腐蚀电位。含0.1 mM Na 2 SnO 3的样品保留了所有样品的最高放电电流密度和最低极化电阻。与其他添加剂浓度相比,混合了0.1 mM Na 2 SnO 3的电解质溶液中的电极具有更大的活性反应面积,更深的通道和更高的放电电流。总之,为了改善Mg-14Li-3Al-1Gd电极在0.7 M NaCl溶液中的电化学行为,Na 2 SnO 3的最佳浓度为0.1 mM。











































 京公网安备 11010802027423号
京公网安备 11010802027423号