当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of All-Carbon Disubstituted Bicyclo[1.1.1]pentanes by Iron-Catalyzed Kumada Cross-Coupling.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-04-28 , DOI: 10.1002/anie.202004090 Jeremy Nugent 1 , Bethany R Shire 1 , Dimitri F J Caputo 1 , Helena D Pickford 1 , Frank Nightingale 1 , Ian T T Houlsby 2 , James J Mousseau 3 , Edward A Anderson 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-04-28 , DOI: 10.1002/anie.202004090 Jeremy Nugent 1 , Bethany R Shire 1 , Dimitri F J Caputo 1 , Helena D Pickford 1 , Frank Nightingale 1 , Ian T T Houlsby 2 , James J Mousseau 3 , Edward A Anderson 1
Affiliation
|
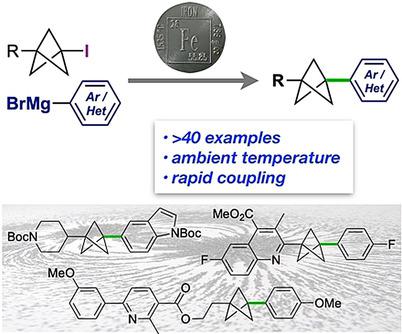
1,3‐Disubstituted bicyclo[1.1.1]pentanes (BCPs) are important motifs in drug design as surrogates for p‐substituted arenes and alkynes. Access to all‐carbon disubstituted BCPs via cross‐coupling has to date been limited to use of the BCP as the organometallic component, which restricts scope due to the harsh conditions typically required for the synthesis of metallated BCPs. Here we report a general method to access 1,3‐C‐disubstituted BCPs from 1‐iodo‐bicyclo[1.1.1]pentanes (iodo‐BCPs) by direct iron‐catalyzed cross‐coupling with aryl and heteroaryl Grignard reagents. This chemistry represents the first general use of iodo‐BCPs as electrophiles in cross‐coupling, and the first Kumada coupling of tertiary iodides. Benefiting from short reaction times, mild conditions, and broad scope of the coupling partners, it enables the synthesis of a wide range of 1,3‐C‐disubstituted BCPs including various drug analogues.
中文翻译:

通过铁催化 Kumada 交叉偶联合成全碳二取代双环[1.1.1]戊烷。
1,3-二取代双环[1.1.1]戊烷(BCP)是药物设计中的重要基序,可作为对位取代芳烃和炔烃的替代物。迄今为止,通过交叉偶联获得全碳二取代的 BCP 仅限于使用 BCP 作为有机金属组分,由于金属化 BCP 的合成通常需要苛刻的条件,这限制了范围。在这里,我们报告了一种通过与芳基和杂芳基格氏试剂直接铁催化交叉偶联从 1-碘-双环[1.1.1]戊烷(碘-BCP)获得 1,3-C-二取代 BCP 的通用方法。这种化学反应代表了碘-BCP 在交叉偶联中作为亲电子试剂的首次普遍应用,以及叔碘化物的首次 Kumada 偶联。受益于短的反应时间、温和的条件和广泛的偶联伙伴,它能够合成各种 1,3- C-二取代的 BCP,包括各种药物类似物。
更新日期:2020-07-06
中文翻译:

通过铁催化 Kumada 交叉偶联合成全碳二取代双环[1.1.1]戊烷。
1,3-二取代双环[1.1.1]戊烷(BCP)是药物设计中的重要基序,可作为对位取代芳烃和炔烃的替代物。迄今为止,通过交叉偶联获得全碳二取代的 BCP 仅限于使用 BCP 作为有机金属组分,由于金属化 BCP 的合成通常需要苛刻的条件,这限制了范围。在这里,我们报告了一种通过与芳基和杂芳基格氏试剂直接铁催化交叉偶联从 1-碘-双环[1.1.1]戊烷(碘-BCP)获得 1,3-C-二取代 BCP 的通用方法。这种化学反应代表了碘-BCP 在交叉偶联中作为亲电子试剂的首次普遍应用,以及叔碘化物的首次 Kumada 偶联。受益于短的反应时间、温和的条件和广泛的偶联伙伴,它能够合成各种 1,3- C-二取代的 BCP,包括各种药物类似物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号