Respiratory Medicine ( IF 3.5 ) Pub Date : 2020-04-29 , DOI: 10.1016/j.rmed.2020.105991 Eric D Bateman 1 , Asif H Khan 2 , Yingxin Xu 3 , Patricia Guyot 2 , Jingdong Chao 3 , Siddhesh Kamat 3 , Paul Rowe 4 , Heather Burnett 5 , Jerome Msihid 4 , David Weinreich 3 , Ian D Pavord 6
|
Background
Currently, five biologic treatment options are available for use in patients with uncontrolled persistent asthma: three interleukin (IL)-5 antagonists, which either bind to the anti-IL-5 ligand (mepolizumab, reslizumab) or to the IL-5 receptor (benralizumab);one anti-immunoglobulin E (anti-IgE) therapy (omalizumab); and one anti-IL-4/IL-13 therapy (dupilumab). To date, no comparative data from head-to-head clinical trials are available for these biologics.
Objective
An indirect treatment comparison (ITC) of dupilumab versus each of the anti-IL-5 and anti-IgE therapies using the endpoints of annualized severe asthma exacerbation rates and change in pre-bronchodilator forced expiratory volume in 1 second (FEV1).
Methods
Embase®, MEDLINE®, and Cochrane Central Register of Controlled Trials (CENTRAL) were searched for studies published between January 1, 1980 and March 25, 2019. Eligible articles included randomized controlled trials (RCTs) in patients aged ≥ 12 years with persistent/uncontrolled asthma using at least medium-to-high dose inhaled corticosteroid plus long-acting β2-agonist with add-on biologic therapy. Bucher ITCs were performed to compare subgroups of dupilumab patients with the anti-IL-5s and anti-IgE trial populations.
Results
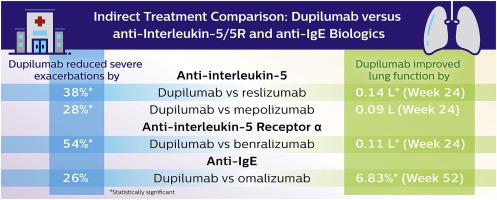
Fourteen RCTs were included in the analyses. The matched dupilumab subgroups were associated with greater reductions in annualized severe exacerbation rates compared with benralizumab, mepolizumab, reslizumab, and omalizumab (54%, 28%, 38%, and 26% greater reduction, respectively). A greater improvement in FEV1 was also observed for dupilumab at week 12 and/or week 24/52 than for the other biologics (0.06–0.14 L).
Conclusion
In this ITC, dupilumab was associated with lower severe asthma exacerbation rates and greater improvements in lung function than anti-IL-5s and omalizumab.
中文翻译:

dupilumab 与其他生物制剂在无法控制的持续性哮喘患者中的成对间接治疗比较
背景
目前,有五种生物治疗方案可用于未控制的持续性哮喘患者:三种白细胞介素 (IL)-5 拮抗剂,可与抗 IL-5 配体(美泊利单抗、瑞利珠单抗)或 IL-5 受体结合。贝那利珠单抗);一种抗免疫球蛋白 E(抗 IgE)疗法(奥马珠单抗);和一种抗 IL-4/IL-13 疗法(dupilumab)。迄今为止,还没有这些生物制剂的头对头临床试验的比较数据。
客观的
dupilumab 与每种抗 IL-5 和抗 IgE 疗法的间接治疗比较 (ITC),使用年化严重哮喘恶化率和 1 秒内支气管扩张剂前用力呼气量 (FEV 1 ) 变化的终点。
方法
检索了 1980 年 1 月 1 日至 2019 年 3 月 25 日期间发表的研究 Embase®、MEDLINE® 和 Cochrane Central Register of Controlled Trials (CENTRAL)。符合条件的文章包括针对年龄 ≥ 12 岁患有持续性/使用至少中高剂量吸入皮质类固醇加长效β 2激动剂和附加生物治疗的未控制哮喘。进行 Bucher ITC 以比较 dupilumab 患者亚组与抗 IL-5 和抗 IgE 试验人群。
结果
分析中包括了 14 项 RCT。与 benralizumab、mepolizumab、reslizumab 和 omalizumab 相比,匹配的 dupilumab 亚组与年化严重恶化率的降低幅度更大(分别降低 54%、28%、38% 和 26%)。在第 12 周和/或第 24/52 周,dupilumab的 FEV 1也比其他生物制剂(0.06-0.14 L)有更大的改善。
结论
在本次 ITC 中,与抗 IL-5 和 omalizumab 相比,dupilumab 与较低的严重哮喘发作率和肺功能的更大改善相关。











































 京公网安备 11010802027423号
京公网安备 11010802027423号