Computational and Theoretical Chemistry ( IF 3.0 ) Pub Date : 2020-04-29 , DOI: 10.1016/j.comptc.2020.112844 Vladimir P. Barannikov , Marina S. Kurbatova , Darya L. Gurina , Nina I. Giricheva
|
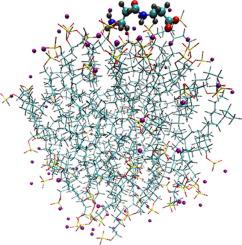
Quantum chemical investigation of α-L-alanyl-α-L-alanine and β-alanyl-β-alanine complexes with SDS dimer as an anion micelle fragment in polarizable continuum has been carried out by using of DFT method with B97-D/6-311++G(3d,3p) level. The variation of peptide structure and the difference in energies of free peptides, SDS dimer and their complexes have been analyzed. Optimized configurations of complexes are stabilized by hydrogen bonds of (H2)N-H…O(S) type. The localization of α-L-alanyl-α-L-alanine among the charged groups of SDS dimer is more preferable. The localization of β-alanyl-β-alanine among the charged groups or in the dimer hydrophpbic canal results in close values of the complex formation energy. More favorite change of energy and shorter length of hydrogen bond of (H2)N-H…O(S) type have been revealed for complex formation between SDS dimer and β-alanyl-β-alanine as opposed to α-L-alanyl-α-L-alanine. Molecular dynamics simulation of adsorption of the peptides on SDS micelle has been performed in NPT ensemble at 0.1 MPa and 298 K. The cell under study contained 14678 water molecules, one peptide zwitter-ion, and the micelle including 64 SDS monomers. Most probable localization of peptide atoms relative the micelle has been analyzed. The peptide – SDS micelle interaction is accompanied by diminish of average number of peptide – water H-bond and the formation of at the mean two H-bonds with SDS. Some peculiarities of adsorption of the isomolecular peptides on SDS micelle are appeared in different values of H-bond lifetimes, different probability of formation of H-bond of NH3+…-O3S-O- or NH3+…-O-(SO3-) types, and localization of peptide zwitter-ions outside or within double electrical layer of the micelle.
中文翻译:

十二烷基硫酸钠胶束与α-L-丙氨酰-α-L-丙氨酸和β-丙氨酰-β-丙氨酸等分子二肽相互作用的量子化学和分子动力学模型
利用B97-D / 6的DFT方法,对极化态连续体中以SDS二聚体为阴离子胶束片段的α-L-丙氨酰-α-L-丙氨酸和β-丙氨酰-β-丙氨酸配合物进行了量子化学研究。 -311 ++ G(3d,3p)级。分析了肽结构的变化以及游离肽,SDS二聚体及其复合物的能量差异。(H 2)NH…O(S)型氢键可稳定配合物的最佳构型。更优选在SDS二聚体的带电基团中α-L-丙氨酰基-α-L-丙氨酸的定位。β-丙氨酸-β-丙氨酸在带电基团之间或在二聚体疏水管中的定位导致复合物形成能的接近值。(H 2)更喜欢的能量变化和较短的氢键长度)NH…O(S)类型已揭示SDS二聚体和β-丙氨酰基-β-丙氨酸与α-L-丙氨酰基-α-L-丙氨酸形成复合物。在NPT集成体中于0.1 MPa和298 K下进行了肽在SDS胶束上吸附的分子动力学模拟。被研究的细胞包含14678个水分子,一个肽两性离子和包含64个SDS单体的胶束。分析了相对于胶束的肽原子最可能的定位。肽与SDS的胶束相互作用伴随着肽与水的H键平均数的减少以及与SDS的平均两个H键的形成。在不同的氢键寿命值,不同的NH 3 +氢键形成概率下,SDS胶束上存在等分子肽吸附的一些特殊性。... - Ô 3 S-O-或NH 3 + ... -O-(SO 3 - )的类型,和肽外部或胶束的双电层内的两性离子的定位。











































 京公网安备 11010802027423号
京公网安备 11010802027423号