Chem ( IF 19.1 ) Pub Date : 2020-04-29 , DOI: 10.1016/j.chempr.2020.04.002 Kuang-Hsu Wu , Dan Wang , Xingyu Lu , Xuefei Zhang , Zailai Xie , Yuefeng Liu , Bing-Jian Su , Jin-Ming Chen , Dang-Sheng Su , Wei Qi , Shaojun Guo
|
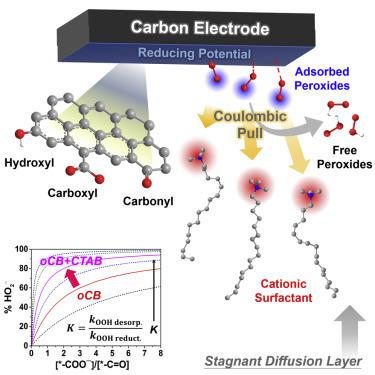
In situ modulation of surface reaction is a powerful approach to drive high-yield H2O2 electrosynthesis on metal-free carbon. Here, we discover that cationic surfactants can work efficiently as an in situ kinetic promoter for the oxygen-to-peroxide reaction on a carbon black electrode, achieving a peroxide yield above 90% (up to 95.2%) across a >0.8 V window in alkaline media, the best among reported H2O2 electrocatalysts. Our characterizations and kinetic model analysis show that the high peroxide selectivity is attributable to surface carboxylates (–COO−) with weak peroxide binding under a Coulombic pull imposed by an adsorbed cationic layer. Although surface carbonyls (–C=O) also participate in the peroxide synthesis, they exhibit strong binding to peroxide and promote on-site reduction at moderate-to-high overpotential. At only a minute amount of cationic surfactant, a chronoamperometry experiment with a carbonyl-free system can deliver a peroxide production at a sustainably high selectivity (∼96%) over 10 h.
中文翻译:

碳上的高选择性过氧化氢电合成:表面活性剂的原位界面工程
表面反应的原位调节是驱动无金属碳上高产率H 2 O 2电合成的有效方法。在这里,我们发现阳离子表面活性剂可以有效地作为原位动力学促进剂,用于炭黑电极上的氧-过氧化物反应,在> 0.8 V的窗口中实现90%以上的过氧化物收率(高达95.2%)。碱性介质,在报道的H 2 O 2电催化剂中最好。我们的表征和动力学模型分析表明,高选择性的过氧化物是由于表面羧酸盐(-COO -)在吸附的阳离子层施加的库仑力作用下具有弱的过氧化物结合力。尽管表面羰基(–C = O)也参与过氧化物的合成,但它们与过氧化物具有很强的结合力,并在中高电位下促进现场还原。仅需极少量的阳离子表面活性剂,使用无羰基体系的计时电流法实验可以在10小时内以持续较高的选择性(〜96%)提供过氧化物的生成。











































 京公网安备 11010802027423号
京公网安备 11010802027423号