当前位置:
X-MOL 学术
›
BBA Mol. Cell Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Protein synthesis inhibition promotes nitric oxide generation and activation of CGKII-dependent downstream signaling pathways in the retina.
Biochimica et Biophysica Acta (BBA) - Molecular Cell Research ( IF 4.6 ) Pub Date : 2020-04-29 , DOI: 10.1016/j.bbamcr.2020.118732 Marcelo Cossenza 1 , Renato Socodato 2 , Telmo A Mejía-García 3 , Ivan Domith 3 , Camila C Portugal 2 , Luis F H Gladulich 3 , Aline T Duarte-Silva 4 , Latika Khatri 5 , Shannon Antoine 6 , Franz Hofmann 7 , Edward B Ziff 5 , Roberto Paes-de-Carvalho 8
Biochimica et Biophysica Acta (BBA) - Molecular Cell Research ( IF 4.6 ) Pub Date : 2020-04-29 , DOI: 10.1016/j.bbamcr.2020.118732 Marcelo Cossenza 1 , Renato Socodato 2 , Telmo A Mejía-García 3 , Ivan Domith 3 , Camila C Portugal 2 , Luis F H Gladulich 3 , Aline T Duarte-Silva 4 , Latika Khatri 5 , Shannon Antoine 6 , Franz Hofmann 7 , Edward B Ziff 5 , Roberto Paes-de-Carvalho 8
Affiliation
|
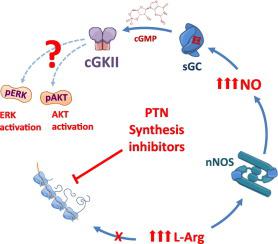
Nitric oxide is an important neuromodulator in the CNS, and its production within neurons is modulated by NMDA receptors and requires a fine-tuned availability of L-arginine. We have previously shown that globally inhibiting protein synthesis mobilizes intracellular L-arginine "pools" in retinal neurons, which concomitantly enhances neuronal nitric oxide synthase-mediated nitric oxide production. Activation of NMDA receptors also induces local inhibition of protein synthesis and L-arginine intracellular accumulation through calcium influx and stimulation of eucariotic elongation factor type 2 kinase. We hypothesized that protein synthesis inhibition might also increase intracellular L-arginine availability to induce nitric oxide-dependent activation of downstream signaling pathways. Here we show that nitric oxide produced by inhibiting protein synthesis (using cycloheximide or anisomycin) is readily coupled to AKT activation in a soluble guanylyl cyclase and cGKII-dependent manner. Knockdown of cGKII prevents cycloheximide or anisomycin-induced AKT activation and its nuclear accumulation. Moreover, in retinas from cGKII knockout mice, cycloheximide was unable to enhance AKT phosphorylation. Indeed, cycloheximide also produces an increase of ERK phosphorylation which is abrogated by a nitric oxide synthase inhibitor. In summary, we show that inhibition of protein synthesis is a previously unanticipated driving force for nitric oxide generation and activation of downstream signaling pathways including AKT and ERK in cultured retinal cells. These results may be important for the regulation of synaptic signaling and neuronal development by NMDA receptors as well as for solving conflicting data observed when using protein synthesis inhibitors for studying neuronal survival during development as well in behavior and memory studies.
中文翻译:

蛋白质合成抑制促进一氧化氮的产生和视网膜中CGKII依赖性下游信号通路的激活。
一氧化氮是CNS中重要的神经调节剂,其在神经元内的产生受NMDA受体的调节,因此需要L-精氨酸的可调节利用率。以前我们已经表明,全局抑制蛋白合成会动员视网膜神经元中的细胞内L-精氨酸“池”,从而增强神经元一氧化氮合酶介导的一氧化氮的产生。NMDA受体的激活还通过钙内流和刺激2型真核生物延伸因子激酶,诱导蛋白质合成和L-精氨酸细胞内积累的局部抑制。我们假设蛋白质合成抑制也可能会增加细胞内L-精氨酸的利用率,以诱导依赖一氧化氮的下游信号通路的激活。在这里,我们显示出通过抑制蛋白质合成(使用环己酰亚胺或茴香霉素)产生的一氧化氮易于以可溶性鸟苷酸环化酶和cGKII依赖性方式与AKT激活偶联。抑制cGKII可防止环己酰亚胺或茴香霉素诱导的AKT活化及其核积累。此外,在来自cGKII基因敲除小鼠的视网膜中,环己酰亚胺无法增强AKT磷酸化。实际上,环己酰亚胺也产生增加的ERK磷酸化,这被一氧化氮合酶抑制剂所消除。总而言之,我们显示蛋白质合成的抑制是一氧化氮的产生和活化的视网膜细胞中下游信号通路(包括AKT和ERK)活化的先前未曾预料的驱动力。
更新日期:2020-04-29
中文翻译:

蛋白质合成抑制促进一氧化氮的产生和视网膜中CGKII依赖性下游信号通路的激活。
一氧化氮是CNS中重要的神经调节剂,其在神经元内的产生受NMDA受体的调节,因此需要L-精氨酸的可调节利用率。以前我们已经表明,全局抑制蛋白合成会动员视网膜神经元中的细胞内L-精氨酸“池”,从而增强神经元一氧化氮合酶介导的一氧化氮的产生。NMDA受体的激活还通过钙内流和刺激2型真核生物延伸因子激酶,诱导蛋白质合成和L-精氨酸细胞内积累的局部抑制。我们假设蛋白质合成抑制也可能会增加细胞内L-精氨酸的利用率,以诱导依赖一氧化氮的下游信号通路的激活。在这里,我们显示出通过抑制蛋白质合成(使用环己酰亚胺或茴香霉素)产生的一氧化氮易于以可溶性鸟苷酸环化酶和cGKII依赖性方式与AKT激活偶联。抑制cGKII可防止环己酰亚胺或茴香霉素诱导的AKT活化及其核积累。此外,在来自cGKII基因敲除小鼠的视网膜中,环己酰亚胺无法增强AKT磷酸化。实际上,环己酰亚胺也产生增加的ERK磷酸化,这被一氧化氮合酶抑制剂所消除。总而言之,我们显示蛋白质合成的抑制是一氧化氮的产生和活化的视网膜细胞中下游信号通路(包括AKT和ERK)活化的先前未曾预料的驱动力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号