当前位置:
X-MOL 学术
›
Chem. Res. Toxicol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Immunochemical Detection of Protein Modification Derived from Metabolic Activation of 8-Epidiosbulbin E Acetate.
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-04-29 , DOI: 10.1021/acs.chemrestox.0c00016 Shenzhi Zhou 1 , Na Zhang 1 , Zixia Hu 1 , Dongju Lin 1 , Weiwei Li 2 , Ying Peng 1 , Jiang Zheng 1, 2, 3
Chemical Research in Toxicology ( IF 3.7 ) Pub Date : 2020-04-29 , DOI: 10.1021/acs.chemrestox.0c00016 Shenzhi Zhou 1 , Na Zhang 1 , Zixia Hu 1 , Dongju Lin 1 , Weiwei Li 2 , Ying Peng 1 , Jiang Zheng 1, 2, 3
Affiliation
|
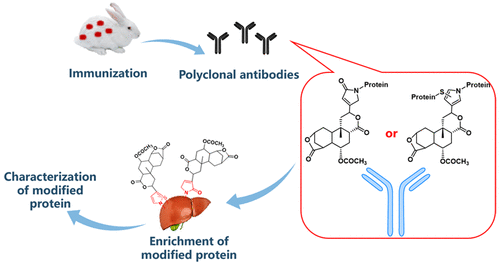
Furanoid 8-epidiosbulbin E acetate (EEA) is one of the most abundant diterpenoid lactones in herbal medicine Dioscorea bulbifera L. (DB). Our early work proved that EEA could be metabolized to EEA-derived cis-enedial (EDE), a reactive intermediate, which is required for the hepatotoxicity observed in experimental animals exposed to EEA. Also, we found that EDE could modify hepatic protein by reaction with thiol groups and/or primary amines of protein. The present study was inclined to develop polyclonal antibodies to detect protein modified by EDE. An immunogen was prepared by reaction of EDE with keyhole limpet hemocyanin (KLH), and polyclonal antibodies were raised in rabbits immunized with the immunogen. Antisera collected from the immunized rabbits demonstrated high titers evaluated by enzyme-linked immunosorbent assays (ELISAs). Immunoblot analysis showed that the polyclonal antibodies recognized EDE-modified bovine serum albumin (BSA) in a hapten load-dependent manner but did not cross-react with native BSA. Competitive inhibition experiments elicited high selectivity of the antibodies toward EDE-modified BSA. The antibodies allowed us to detect and enrich EDE-modified protein in liver homogenates obtained from EEA-treated mice. The developed immunoprecipitation technique, along with mass spectrometry, enabled us to succeed in identifying multiple hepatic proteins of animals given EEA. We have successfully developed polyclonal antibodies with the ability to recognize EDE-derived protein adducts, which is a unique tool for us to define the mechanisms of toxic action of EEA.
中文翻译:

蛋白质化学修饰的免疫化学检测,它是由8-Epidiosbulbin E Acetate的代谢活化引起的。
呋喃酮8-上表皮素E乙酸酯(EEA)是草药Dioscorea bulbifera L.(DB)中含量最丰富的二萜类内酯之一。我们的早期工作证明EEA可以代谢为EEA衍生的顺式-烯醛(EDE),一种反应性中间体,是暴露于EEA的实验动物所观察到的肝毒性所必需的。此外,我们发现EDE可以通过与巯基和/或蛋白质的伯胺反应来修饰肝脏蛋白质。本研究倾向于开发多克隆抗体以检测被EDE修饰的蛋白质。通过EDE与匙孔血蓝蛋白(KLH)反应制备免疫原,并在用该免疫原免疫的兔子中产生多克隆抗体。从经免疫的兔子收集的抗血清显示出通过酶联免疫吸附测定(ELISA)评估的高滴度。免疫印迹分析表明,多克隆抗体以半抗原依赖的方式识别EDE修饰的牛血清白蛋白(BSA),但不与天然BSA交叉反应。竞争性抑制实验引起抗体对EDE修饰的BSA的高选择性。抗体使我们能够检测和富集从EEA处理的小鼠肝脏匀浆中的EDE修饰蛋白。发达的免疫沉淀技术以及质谱技术使我们能够成功鉴定出使用EEA的动物的多种肝蛋白。我们已经成功开发出了具有识别EDE衍生的蛋白质加合物的能力的多克隆抗体,这是我们定义EEA毒性作用机制的独特工具。使我们能够成功鉴定出给予EEA的动物多种肝蛋白。我们已经成功开发出了具有识别EDE衍生的蛋白质加合物的能力的多克隆抗体,这是我们定义EEA毒性作用机制的独特工具。使我们能够成功鉴定出给予EEA的动物多种肝蛋白。我们已经成功开发出了具有识别EDE衍生的蛋白质加合物的能力的多克隆抗体,这是我们定义EEA毒性作用机制的独特工具。
更新日期:2020-04-29
中文翻译:

蛋白质化学修饰的免疫化学检测,它是由8-Epidiosbulbin E Acetate的代谢活化引起的。
呋喃酮8-上表皮素E乙酸酯(EEA)是草药Dioscorea bulbifera L.(DB)中含量最丰富的二萜类内酯之一。我们的早期工作证明EEA可以代谢为EEA衍生的顺式-烯醛(EDE),一种反应性中间体,是暴露于EEA的实验动物所观察到的肝毒性所必需的。此外,我们发现EDE可以通过与巯基和/或蛋白质的伯胺反应来修饰肝脏蛋白质。本研究倾向于开发多克隆抗体以检测被EDE修饰的蛋白质。通过EDE与匙孔血蓝蛋白(KLH)反应制备免疫原,并在用该免疫原免疫的兔子中产生多克隆抗体。从经免疫的兔子收集的抗血清显示出通过酶联免疫吸附测定(ELISA)评估的高滴度。免疫印迹分析表明,多克隆抗体以半抗原依赖的方式识别EDE修饰的牛血清白蛋白(BSA),但不与天然BSA交叉反应。竞争性抑制实验引起抗体对EDE修饰的BSA的高选择性。抗体使我们能够检测和富集从EEA处理的小鼠肝脏匀浆中的EDE修饰蛋白。发达的免疫沉淀技术以及质谱技术使我们能够成功鉴定出使用EEA的动物的多种肝蛋白。我们已经成功开发出了具有识别EDE衍生的蛋白质加合物的能力的多克隆抗体,这是我们定义EEA毒性作用机制的独特工具。使我们能够成功鉴定出给予EEA的动物多种肝蛋白。我们已经成功开发出了具有识别EDE衍生的蛋白质加合物的能力的多克隆抗体,这是我们定义EEA毒性作用机制的独特工具。使我们能够成功鉴定出给予EEA的动物多种肝蛋白。我们已经成功开发出了具有识别EDE衍生的蛋白质加合物的能力的多克隆抗体,这是我们定义EEA毒性作用机制的独特工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号