当前位置:
X-MOL 学术
›
Eur. J. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cytoplasmic localization of amyotrophic lateral sclerosis-related TDP-43 proteins modulates stress granule formation.
European Journal of Neuroscience ( IF 2.7 ) Pub Date : 2020-04-28 , DOI: 10.1111/ejn.14762 Corinne Besnard-Guérin 1
European Journal of Neuroscience ( IF 2.7 ) Pub Date : 2020-04-28 , DOI: 10.1111/ejn.14762 Corinne Besnard-Guérin 1
Affiliation
|
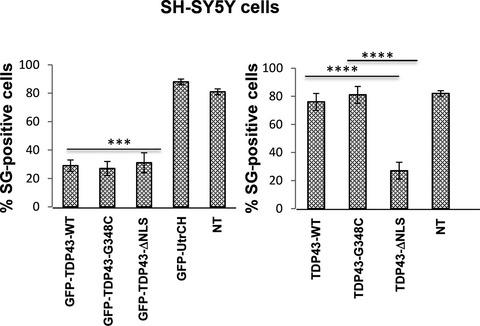
TDP‐43 is an RNA/DNA‐binding protein associated with amyotrophic lateral sclerosis (ALS). Under pathological conditions, TDP‐43 exported from the nucleus accumulates in the cytoplasm, forming inclusion bodies. However, the molecular mechanisms that contribute to such aggregation are unclear. The pathogenic processes that lead to aggregation in ALS were investigated by analysing the effects of wildtype human TDP‐43 or with mutations in the nuclear localization sequence (NLS) or those associated with ALS in stress granule formation. TDP‐43 (WT, ∆NLS or G348C), with or without a GFP‐tag, was expressed in SH‐SY5Y neuroblastoma or HeLa cells and stress granules induced by oxidative stress or heat shock. Stress granule formation was altered in cells strongly expressing GFP‐TDP‐∆NLS, or untagged TDP‐43‐∆NLS in the cytoplasm but not the negative controls, GFP or GFP‐UtrCH. In contrast, there was no reduction in stress granule formation by cells that expressed untagged TDP‐43 (WT or G348C) in the nucleus upon stress induction. GFP labelling of TDP‐43 (WT or G348C) promotes high cytoplasmic expression and nuclear aggregation. Stress granule formation was impaired in cells expressing GFP‐TDP‐43 (WT or G348C) in the cytoplasm. Overall, these results suggest that stress granule formation may be inhibited by high levels of TDP‐43 protein in the cytoplasm. As stress granules serve a protective function, their deregulation may promote neurodegeneration due to an aberrant stress response.
中文翻译:

肌萎缩侧索硬化相关 TDP-43 蛋白的细胞质定位调节应激颗粒的形成。
TDP-43 是一种与肌萎缩侧索硬化症 (ALS) 相关的 RNA/DNA 结合蛋白。在病理条件下,从细胞核输出的TDP-43在细胞质中积累,形成包涵体。然而,导致这种聚集的分子机制尚不清楚。通过分析野生型人类 TDP-43 或核定位序列 (NLS) 突变或与 ALS 在应力颗粒形成中相关的突变的影响,研究了导致 ALS 聚集的致病过程。TDP-43(WT、ΔNLS 或 G348C),带有或不带有 GFP 标签,在氧化应激或热休克诱导的 SH-SY5Y 神经母细胞瘤或 HeLa 细胞和应激颗粒中表达。在细胞质中强烈表达 GFP-TDP-ΔNLS 或未标记的 TDP-43-ΔNLS 的细胞中,应力颗粒的形成发生了改变,而阴性对照则没有,GFP 或 GFP-UtrCH。相比之下,在应激诱导时,细胞核中表达未标记 TDP-43(WT 或 G348C)的细胞的应激颗粒形成没有减少。TDP-43(WT 或 G348C)的 GFP 标记促进高细胞质表达和核聚集。在细胞质中表达 GFP-TDP-43(WT 或 G348C)的细胞中,应激颗粒的形成受损。总体而言,这些结果表明,细胞质中高水平的 TDP-43 蛋白可能会抑制应激颗粒的形成。由于应激颗粒具有保护功能,它们的失调可能会由于异常的应激反应而促进神经退行性变。TDP-43(WT 或 G348C)的 GFP 标记促进高细胞质表达和核聚集。在细胞质中表达 GFP-TDP-43(WT 或 G348C)的细胞中,应激颗粒的形成受损。总体而言,这些结果表明,细胞质中高水平的 TDP-43 蛋白可能会抑制应激颗粒的形成。由于应激颗粒具有保护功能,它们的失调可能会由于异常的应激反应而促进神经退行性变。TDP-43(WT 或 G348C)的 GFP 标记促进高细胞质表达和核聚集。在细胞质中表达 GFP-TDP-43(WT 或 G348C)的细胞中,应激颗粒的形成受损。总体而言,这些结果表明,细胞质中高水平的 TDP-43 蛋白可能会抑制应激颗粒的形成。由于应激颗粒具有保护功能,它们的失调可能会由于异常的应激反应而促进神经退行性变。
更新日期:2020-04-28
中文翻译:

肌萎缩侧索硬化相关 TDP-43 蛋白的细胞质定位调节应激颗粒的形成。
TDP-43 是一种与肌萎缩侧索硬化症 (ALS) 相关的 RNA/DNA 结合蛋白。在病理条件下,从细胞核输出的TDP-43在细胞质中积累,形成包涵体。然而,导致这种聚集的分子机制尚不清楚。通过分析野生型人类 TDP-43 或核定位序列 (NLS) 突变或与 ALS 在应力颗粒形成中相关的突变的影响,研究了导致 ALS 聚集的致病过程。TDP-43(WT、ΔNLS 或 G348C),带有或不带有 GFP 标签,在氧化应激或热休克诱导的 SH-SY5Y 神经母细胞瘤或 HeLa 细胞和应激颗粒中表达。在细胞质中强烈表达 GFP-TDP-ΔNLS 或未标记的 TDP-43-ΔNLS 的细胞中,应力颗粒的形成发生了改变,而阴性对照则没有,GFP 或 GFP-UtrCH。相比之下,在应激诱导时,细胞核中表达未标记 TDP-43(WT 或 G348C)的细胞的应激颗粒形成没有减少。TDP-43(WT 或 G348C)的 GFP 标记促进高细胞质表达和核聚集。在细胞质中表达 GFP-TDP-43(WT 或 G348C)的细胞中,应激颗粒的形成受损。总体而言,这些结果表明,细胞质中高水平的 TDP-43 蛋白可能会抑制应激颗粒的形成。由于应激颗粒具有保护功能,它们的失调可能会由于异常的应激反应而促进神经退行性变。TDP-43(WT 或 G348C)的 GFP 标记促进高细胞质表达和核聚集。在细胞质中表达 GFP-TDP-43(WT 或 G348C)的细胞中,应激颗粒的形成受损。总体而言,这些结果表明,细胞质中高水平的 TDP-43 蛋白可能会抑制应激颗粒的形成。由于应激颗粒具有保护功能,它们的失调可能会由于异常的应激反应而促进神经退行性变。TDP-43(WT 或 G348C)的 GFP 标记促进高细胞质表达和核聚集。在细胞质中表达 GFP-TDP-43(WT 或 G348C)的细胞中,应激颗粒的形成受损。总体而言,这些结果表明,细胞质中高水平的 TDP-43 蛋白可能会抑制应激颗粒的形成。由于应激颗粒具有保护功能,它们的失调可能会由于异常的应激反应而促进神经退行性变。











































 京公网安备 11010802027423号
京公网安备 11010802027423号