当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Efficient Asymmetric Synthesis of Ethyl (S)-4-Chloro-3-hydroxybutyrate Using Alcohol Dehydrogenase SmADH31 with High Tolerance of Substrate and Product in a Monophasic Aqueous System
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-04-28 , DOI: 10.1021/acs.oprd.0c00088 Zeyu Yang 1 , Wenjie Ye 1 , Youyu Xie 1 , Qinghai Liu 1 , Rong Chen 2 , Hualei Wang 1 , Dongzhi Wei 1
Organic Process Research & Development ( IF 3.4 ) Pub Date : 2020-04-28 , DOI: 10.1021/acs.oprd.0c00088 Zeyu Yang 1 , Wenjie Ye 1 , Youyu Xie 1 , Qinghai Liu 1 , Rong Chen 2 , Hualei Wang 1 , Dongzhi Wei 1
Affiliation
|
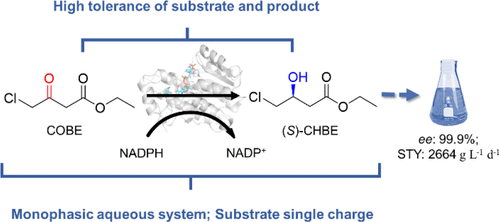
Bioreductions catalyzed by alcohol dehydrogenases (ADHs) play an important role in the synthesis of chiral alcohols. However, the synthesis of ethyl (S)-4-chloro-3-hydroxybutyrate [(S)-CHBE], an important drug intermediate, has significant challenges concerning high substrate or product inhibition toward ADHs, which complicates its production. Herein, we evaluated a novel ADH, SmADH31, obtained from the Stenotrophomonas maltophilia genome, which can tolerate extremely high concentrations (6 M) of both substrate and product. The coexpression of SmADH31 and glucose dehydrogenase from Bacillus subtilis in Escherichia coli meant that as much as 660 g L–1 (4.0 M) ethyl 4-chloroacetoacetate was completely converted into (S)-CHBE in a monophasic aqueous system with a >99.9% ee value and a high space-time yield (2664 g L–1 d–1). Molecular dynamics simulation shed light on the high activity and stereoselectivity of SmADH31. Moreover, five other optically pure chiral alcohols were synthesized at high concentrations (100–462 g L–1) as a result of the broad substrate spectrum of SmADH31. All these compounds act as important drug intermediates, demonstrating the industrial potential of SmADH31-mediated bioreductions.
中文翻译:

单相水体系中具有高耐受性的底物和产物的醇脱氢酶Sm ADH31有效地不对称合成(S)-4-氯-3-羟基丁酸乙酯
醇脱氢酶(ADHs)催化的生物还原在手性醇的合成中起重要作用。然而,重要的药物中间体((S)-4-氯-3-羟基丁酸乙酯([ S)-CHBE])的合成对于高底物或产物对ADH的抑制作用具有重大挑战,这使其生产变得复杂。本文中,我们评估了一种新的ADH,即Sm ADH31,它从嗜麦芽单胞菌的嗜麦芽孢杆菌基因组中获得,其可以耐受极高浓度(6 M)的底物和产物。的共表达的Sm ADH31和葡萄糖脱氢酶从枯草芽孢杆菌在大肠杆菌意味着高达660克L-在单相水性体系中,–1(4.0 M)4-氯乙酰乙酸乙酯已完全转化为(S)-CHBE,其ee值> 99.9%,时空产率高(2664 g L –1 d –1)。分子动力学模拟揭示了Sm ADH31的高活性和立体选择性。此外,由于Sm ADH31具有较宽的底物谱,因此还以高浓度(100–462 g L –1)合成了其他五种光学纯手性醇。所有这些化合物均充当重要的药物中间体,证明了Sm ADH31介导的生物还原的工业潜力。
更新日期:2020-06-19
中文翻译:

单相水体系中具有高耐受性的底物和产物的醇脱氢酶Sm ADH31有效地不对称合成(S)-4-氯-3-羟基丁酸乙酯
醇脱氢酶(ADHs)催化的生物还原在手性醇的合成中起重要作用。然而,重要的药物中间体((S)-4-氯-3-羟基丁酸乙酯([ S)-CHBE])的合成对于高底物或产物对ADH的抑制作用具有重大挑战,这使其生产变得复杂。本文中,我们评估了一种新的ADH,即Sm ADH31,它从嗜麦芽单胞菌的嗜麦芽孢杆菌基因组中获得,其可以耐受极高浓度(6 M)的底物和产物。的共表达的Sm ADH31和葡萄糖脱氢酶从枯草芽孢杆菌在大肠杆菌意味着高达660克L-在单相水性体系中,–1(4.0 M)4-氯乙酰乙酸乙酯已完全转化为(S)-CHBE,其ee值> 99.9%,时空产率高(2664 g L –1 d –1)。分子动力学模拟揭示了Sm ADH31的高活性和立体选择性。此外,由于Sm ADH31具有较宽的底物谱,因此还以高浓度(100–462 g L –1)合成了其他五种光学纯手性醇。所有这些化合物均充当重要的药物中间体,证明了Sm ADH31介导的生物还原的工业潜力。



























 京公网安备 11010802027423号
京公网安备 11010802027423号