当前位置:
X-MOL 学术
›
ACS Infect. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Resistome of Staphylococcus aureus in Response to Human Cathelicidin LL-37 and Its Engineered Antimicrobial Peptides.
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2020-04-28 , DOI: 10.1021/acsinfecdis.0c00112 Radha M Golla 1 , Biswajit Mishra 1 , Xiangli Dang 1 , Jayaram Lakshmaiah Narayana 1 , Amy Li 2 , Libin Xu 2 , Guangshun Wang 1
ACS Infectious Diseases ( IF 4.0 ) Pub Date : 2020-04-28 , DOI: 10.1021/acsinfecdis.0c00112 Radha M Golla 1 , Biswajit Mishra 1 , Xiangli Dang 1 , Jayaram Lakshmaiah Narayana 1 , Amy Li 2 , Libin Xu 2 , Guangshun Wang 1
Affiliation
|
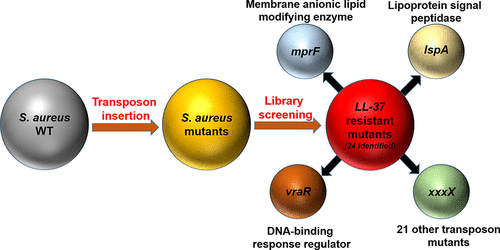
Staphylococcus aureus is notoriously known for its rapid development of resistance to conventional antibiotics. S. aureus can alter its membrane composition to reduce the killing effect of antibiotics and antimicrobial peptides (AMPs). To obtain a more complete picture, this study identified the resistance genes of S. aureus in response to human cathelicidin LL-37 peptides by screening the Nebraska Transposon Mutant Library. In total, 24 resistant genes were identified. Among them, six mutants, including the one with the known membrane-modifying gene (mprF) disabled, became more membrane permeable to the LL-37 engineered peptide 17BIPHE2 than the wild type. Mass spectrometry analysis detected minimal lysyl-phosphatidylglycerol (lysylPG) from the mprF mutant of S. aureus JE2, confirming loss-of-function of this gene. Moreover, multiple mutants showed reduced surface adhesion and biofilm formation. In addition, four S. aureus mutants were unable to infect wax moth Galleria mellonella. There appears to be a connection between the ability of bacterial attachment/biofilm formation and infection. These results underscore the multiple functional roles of the identified peptide-response genes in bacterial growth, infection, and biofilm formation. Therefore, S. aureus utilizes a set of resistant genes to weave a complex molecular network to handle the danger posed by cationic LL-37. It appears that different genes are involved depending on the nature of antimicrobials. These resistant genes may offer a novel avenue to designing more potent antibiotics that target the Achilles heels of S. aureus USA300, a community-associated pathogen of great threat.
中文翻译:

金黄色葡萄球菌对人Cathelicidin LL-37及其工程抗菌肽的抗性
众所周知,金黄色葡萄球菌以其对常规抗生素的耐药性迅速发展而闻名。金黄色葡萄球菌可以改变其膜组成,以降低抗生素和抗菌肽(AMP)的杀灭作用。为了获得更完整的图片,本研究通过筛选内布拉斯加转座子突变体文库,鉴定了金黄色葡萄球菌对人cathelicidin LL-37肽的抗性基因。总共鉴定出24个抗性基因。其中,六个突变体,包括一个具有已知的膜修饰基因(mprF)被禁用的突变体,比野生型对LL-37工程肽17BIPHE2的膜通透性更高。质谱分析检测到的微量赖氨酰磷脂酰甘油(lysylPG)来自金黄色葡萄球菌JE2的mprF突变体,证实了该基因的功能丧失。此外,多个突变体显示减少的表面粘附和生物膜形成。此外,四个金黄色葡萄球菌突变体无法感染蜡蛾蜡螟。细菌附着/生物膜形成的能力与感染之间似乎存在联系。这些结果强调了已鉴定的肽反应基因在细菌生长,感染和生物膜形成中的多种功能作用。因此,金黄色葡萄球菌利用一组抗性基因编织一个复杂的分子网络来应对阳离子LL-37带来的危险。似乎取决于抗微生物剂的性质涉及不同的基因。这些抗性基因可能为设计更有效的抗生素提供了一条新途径,这些抗生素可以针对金黄色葡萄球菌USA300(与社区相关的具有巨大威胁的病原体)的跟腱。
更新日期:2020-04-28
中文翻译:

金黄色葡萄球菌对人Cathelicidin LL-37及其工程抗菌肽的抗性
众所周知,金黄色葡萄球菌以其对常规抗生素的耐药性迅速发展而闻名。金黄色葡萄球菌可以改变其膜组成,以降低抗生素和抗菌肽(AMP)的杀灭作用。为了获得更完整的图片,本研究通过筛选内布拉斯加转座子突变体文库,鉴定了金黄色葡萄球菌对人cathelicidin LL-37肽的抗性基因。总共鉴定出24个抗性基因。其中,六个突变体,包括一个具有已知的膜修饰基因(mprF)被禁用的突变体,比野生型对LL-37工程肽17BIPHE2的膜通透性更高。质谱分析检测到的微量赖氨酰磷脂酰甘油(lysylPG)来自金黄色葡萄球菌JE2的mprF突变体,证实了该基因的功能丧失。此外,多个突变体显示减少的表面粘附和生物膜形成。此外,四个金黄色葡萄球菌突变体无法感染蜡蛾蜡螟。细菌附着/生物膜形成的能力与感染之间似乎存在联系。这些结果强调了已鉴定的肽反应基因在细菌生长,感染和生物膜形成中的多种功能作用。因此,金黄色葡萄球菌利用一组抗性基因编织一个复杂的分子网络来应对阳离子LL-37带来的危险。似乎取决于抗微生物剂的性质涉及不同的基因。这些抗性基因可能为设计更有效的抗生素提供了一条新途径,这些抗生素可以针对金黄色葡萄球菌USA300(与社区相关的具有巨大威胁的病原体)的跟腱。











































 京公网安备 11010802027423号
京公网安备 11010802027423号