当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unifying the Aminohexopyranose- and Peptidyl-Nucleoside Antibiotics: Implications for Antibiotic Design.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-04-27 , DOI: 10.1002/anie.202003094 Catherine M Serrano 1 , Hariprasada Reddy Kanna Reddy 1 , Daniel Eiler 2 , Michael Koch 3 , Ben I C Tresco 1 , Louis R Barrows 3 , Ryan T VanderLinden 4 , Charles A Testa 4 , Paul R Sebahar 4 , Ryan E Looper 1, 4
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2020-04-27 , DOI: 10.1002/anie.202003094 Catherine M Serrano 1 , Hariprasada Reddy Kanna Reddy 1 , Daniel Eiler 2 , Michael Koch 3 , Ben I C Tresco 1 , Louis R Barrows 3 , Ryan T VanderLinden 4 , Charles A Testa 4 , Paul R Sebahar 4 , Ryan E Looper 1, 4
Affiliation
|
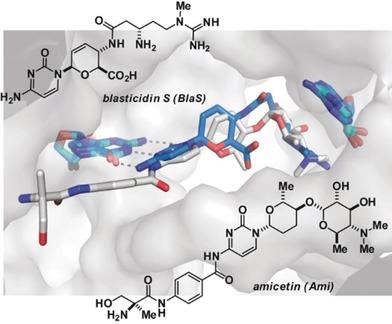
In search of new anti‐tuberculars compatible with anti‐retroviral therapy we re‐identified amicetin as a lead compound. Amicetin's binding to the 70S ribosomal subunit of Thermus thermophilus (Tth ) has been unambiguously determined by crystallography and reveals it to occupy the peptidyl transferase center P‐site of the ribosome. The amicetin binding site overlaps significantly with that of the well‐known protein synthesis inhibitor balsticidin S. Amicetin, however, is the first compound structurally characterized to bind to the P‐site with demonstrated selectivity for the inhibition of prokaryotic translation. The natural product‐ribosome structure enabled the synthesis of simplified analogues that retained both potency and selectivity for the inhibition of prokaryotic translation.
中文翻译:

统一吡喃氨基己糖和肽基核苷抗生素:对抗生素设计的影响。
为了寻找与抗逆转录病毒治疗相容的新型抗结核药物,我们重新确定了阿米西丁作为先导化合物。 Amicetin 与嗜热栖热菌( Tth ) 70S 核糖体亚基的结合已通过晶体学明确确定,并揭示其占据核糖体的肽基转移酶中心 P 位点。 Amicetin 的结合位点与著名的蛋白质合成抑制剂 balsticidin S 的结合位点显着重叠。然而,Amicetin 是第一个在结构上与 P 位点结合的化合物,并显示出对原核翻译抑制的选择性。天然产物核糖体结构使得能够合成简化的类似物,保留抑制原核翻译的效力和选择性。
更新日期:2020-07-01
中文翻译:

统一吡喃氨基己糖和肽基核苷抗生素:对抗生素设计的影响。
为了寻找与抗逆转录病毒治疗相容的新型抗结核药物,我们重新确定了阿米西丁作为先导化合物。 Amicetin 与嗜热栖热菌( Tth ) 70S 核糖体亚基的结合已通过晶体学明确确定,并揭示其占据核糖体的肽基转移酶中心 P 位点。 Amicetin 的结合位点与著名的蛋白质合成抑制剂 balsticidin S 的结合位点显着重叠。然而,Amicetin 是第一个在结构上与 P 位点结合的化合物,并显示出对原核翻译抑制的选择性。天然产物核糖体结构使得能够合成简化的类似物,保留抑制原核翻译的效力和选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号