当前位置:
X-MOL 学术
›
Inorg. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phosphine-substituted Fe–Te clusters related to the active site of [FeFe]-H2ases
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2020-04-28 , DOI: 10.1039/d0qi00276c Shuang Lü 1, 2, 3, 4 , Hong-Li Huang 2, 4, 5, 6, 7 , Ru-fen Zhang 2, 4, 5, 6, 7 , Chun-lin Ma 2, 4, 5, 6, 7 , Qian-Li Li 2, 4, 5, 6, 7 , Jiao He 4, 8, 9, 10 , Jun Yang 4, 8, 9, 10 , Ting Li 4, 8, 9, 10 , Yu-Long Li 4, 8, 9, 10, 11
Inorganic Chemistry Frontiers ( IF 6.1 ) Pub Date : 2020-04-28 , DOI: 10.1039/d0qi00276c Shuang Lü 1, 2, 3, 4 , Hong-Li Huang 2, 4, 5, 6, 7 , Ru-fen Zhang 2, 4, 5, 6, 7 , Chun-lin Ma 2, 4, 5, 6, 7 , Qian-Li Li 2, 4, 5, 6, 7 , Jiao He 4, 8, 9, 10 , Jun Yang 4, 8, 9, 10 , Ting Li 4, 8, 9, 10 , Yu-Long Li 4, 8, 9, 10, 11
Affiliation
|
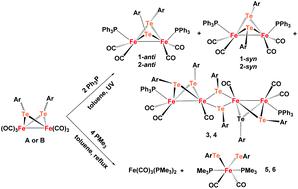
In order to enrich the chemistry of the Fe/Te cluster compounds related to the active site of the [FeFe]-hydrogenase, the reactions of compound Fe2(μ-TeAr)2(CO)6 with tertiary phosphines were described. Three types of novel phosphine substituted Fe/Te model compounds were successfully prepared. That is, the treatment of Fe2(μ-TeAr)2(CO)6 (Ar = C6H5A, C6H4-F-4 B) with 2 equivalents of PPh3 under UV conditions produced dinuclear compounds Fe2(μ-TeC6H5)2(CO)4(PPh3)2 (1) and Fe2(μ-TeC6H4-F-4)2(CO)4(PPh3)2 (2) in moderate yields together with unusual tetranuclear compounds Fe4(μ-TeC6H5)6(CO)6(PPh3)2 (3) and Fe4(μ-TeC6H4-4-F)6(CO)6(PPh3)2 (4). The syn- and anti-isomers of 1 and 2 were isolated in the solid state using the TLC method. Furthermore, the reaction of Fe2(μ-TeAr)2(CO)6 and 4 equivalents of PMe3 gave new types of mononuclear PMe3-disubstituted compounds Fe(TeC6H5)2(CO)2(PMe3)2 (5) and Fe(TeC6H4-4-F)2(CO)2(PMe3)2 (6) which could mimic Fed of the [FeFe]-hydrogenase. All the new compounds were fully characterized by elemental analysis, IR, NMR spectroscopy, UV-Visible absorption and particularly some of them by X-ray crystallography. The electrochemical properties of 1, 2, 5 and 6 were also investigated, demonstrating that these compounds can be considered as electrochemical catalysts for the hydrogen evolution reaction. According to the electrochemical behavior of 5 and 6, a CEECC mechanism was proposed for the catalytic process.
中文翻译:

膦取代的Fe–Te簇与[FeFe] -H2ase的活性位点有关
为了丰富与[FeFe]加氢酶活性位点有关的Fe / Te簇化合物的化学性质,描述了化合物Fe 2(μ-TeAr)2(CO)6与叔膦的反应。成功制备了三种新型的膦取代的Fe / Te模型化合物。即,在紫外线条件下用2当量的PPh 3处理Fe 2(μ-TeAr)2(CO)6(Ar = C 6 H 5 A,C 6 H 4 -F-4 B)产生双核化合物Fe 2(μ-TEC 6 ħ 5)2(CO)4(PPH 3)2(1)和Fe 2(μ-TEC 6 ħ 4 -F-4)2(CO)4(PPH 3)2(2)在中等产率与不寻常的四环化合物一起的Fe 4(μ-TEC 6 ħ 5)6(CO)6(PPH 3)2(3)和Fe 4(μ-TEC 6 ħ 4 -4-F)6(CO)6(PPh 3)2(4)。使用TLC方法以固态分离出1和2的顺式和反式异构体。此外,Fe 2(μ-TeAr)2(CO)6和4当量的PMe 3的反应产生了新型单核PMe 3-二取代化合物Fe(TeC 6 H 5)2(CO)2(PMe 3)2(5)和Fe(TeC 6 H 4 -4-F)2(CO)2(PMe 3)2(6)可以模拟[FeFe]氢化酶的Fe d。所有新化合物均通过元素分析,红外光谱,核磁共振谱,紫外可见吸收剂进行了充分表征,尤其是其中一些通过X射线晶体学进行了表征。的电化学性质1,2,5和6进行了研究,表明这些化合物可以被认为是用于析氢反应的电化学催化剂。根据5和6的电化学行为,提出了CEECC催化过程的机理。
更新日期:2020-04-28
中文翻译:

膦取代的Fe–Te簇与[FeFe] -H2ase的活性位点有关
为了丰富与[FeFe]加氢酶活性位点有关的Fe / Te簇化合物的化学性质,描述了化合物Fe 2(μ-TeAr)2(CO)6与叔膦的反应。成功制备了三种新型的膦取代的Fe / Te模型化合物。即,在紫外线条件下用2当量的PPh 3处理Fe 2(μ-TeAr)2(CO)6(Ar = C 6 H 5 A,C 6 H 4 -F-4 B)产生双核化合物Fe 2(μ-TEC 6 ħ 5)2(CO)4(PPH 3)2(1)和Fe 2(μ-TEC 6 ħ 4 -F-4)2(CO)4(PPH 3)2(2)在中等产率与不寻常的四环化合物一起的Fe 4(μ-TEC 6 ħ 5)6(CO)6(PPH 3)2(3)和Fe 4(μ-TEC 6 ħ 4 -4-F)6(CO)6(PPh 3)2(4)。使用TLC方法以固态分离出1和2的顺式和反式异构体。此外,Fe 2(μ-TeAr)2(CO)6和4当量的PMe 3的反应产生了新型单核PMe 3-二取代化合物Fe(TeC 6 H 5)2(CO)2(PMe 3)2(5)和Fe(TeC 6 H 4 -4-F)2(CO)2(PMe 3)2(6)可以模拟[FeFe]氢化酶的Fe d。所有新化合物均通过元素分析,红外光谱,核磁共振谱,紫外可见吸收剂进行了充分表征,尤其是其中一些通过X射线晶体学进行了表征。的电化学性质1,2,5和6进行了研究,表明这些化合物可以被认为是用于析氢反应的电化学催化剂。根据5和6的电化学行为,提出了CEECC催化过程的机理。









































 京公网安备 11010802027423号
京公网安备 11010802027423号