Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
StpA represses CRISPR-Cas immunity in H-NS deficient Escherichia coli.
Biochimie ( IF 3.9 ) Pub Date : 2020-04-28 , DOI: 10.1016/j.biochi.2020.04.020 Damjan Mitić 1 , Marin Radovčić 1 , Dora Markulin 1 , Ivana Ivančić-Baće 1
Biochimie ( IF 3.9 ) Pub Date : 2020-04-28 , DOI: 10.1016/j.biochi.2020.04.020 Damjan Mitić 1 , Marin Radovčić 1 , Dora Markulin 1 , Ivana Ivančić-Baće 1
Affiliation
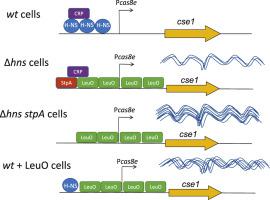
|
Functional CRISPR-Cas systems provide many bacteria and most archaea with adaptive immunity against invading DNA elements. CRISPR arrays store DNA fragments of previous infections while products of cas genes provide immunity by integrating new DNA fragments and using this information to recognize and destroy invading DNA. Escherichia coli contains the CRISPR-Cas type I-E system in which foreign DNA targets are recognized by Cascade, a crRNA-guided complex comprising five proteins (CasA, CasB, CasC, CasD, CasE), and degraded by Cas3. In E. coli the CRISPR-Cas type I-E system is repressed by the histone-like nucleoid-structuring protein H-NS. H-NS repression can be relieved either by inactivation of the hns gene or by elevated levels of the H-NS antagonist LeuO, which induces higher transcript levels of cas genes than was observed for Δhns cells. This suggests that derepression in Δhns cells is incomplete and that an additional repressor could be involved in the silencing. One such candidate is the H-NS paralog protein StpA, which has DNA binding preferences similar to those of H-NS. Here we show that overexpression of StpA in Δhns cells containing anti-lambda spacers abolishes resistance to λvir infection and reduces transcription of the casA gene. In cells lacking hns and stpA genes, the transcript levels of the casA gene are higher than Δhns and similar to wt cells overexpressing LeuO. Taken together, these results suggest that Cascade genes in E. coli are repressed by the StpA protein when H-NS is absent.
中文翻译:

StpA抑制H-NS缺陷型大肠杆菌中的CRISPR-Cas免疫。
功能性CRISPR-Cas系统为许多细菌和大多数古细菌提供了针对入侵DNA元素的适应性免疫力。CRISPR阵列可存储先前感染的DNA片段,而cas基因产物可通过整合新的DNA片段并利用此信息识别和破坏入侵的DNA来提供免疫力。大肠杆菌包含CRISPR-Cas型IE系统,其中外源DNA靶点被Cascade识别,Cascade是由crRNA引导的复合物,包含5种蛋白质(CasA,CasB,CasC,CasD,CasE),并被Cas3降解。在大肠杆菌中,CRISPR-Cas型IE系统被组蛋白样核糖结构蛋白H-NS抑制。H-NS抑制可通过hns基因失活或升高水平的H-NS拮抗剂LeuO缓解,后者诱导cas基因的转录水平高于Δhns细胞。这表明Δhns细胞中的阻抑作用是不完全的,另外的阻遏物可能参与沉默。一种这样的候选物是H-NS旁系同源蛋白StpA,其具有与H-NS相似的DNA结合偏好。在这里,我们显示StpA在含有抗λ间隔子的Δhns细胞中的过表达消除了对λvir感染的抵抗力,并减少了casA基因的转录。在缺乏hns和stpA基因的细胞中,casA基因的转录水平高于Δhns,与过表达LeuO的wt细胞相似。综上,这些结果表明,当H-NS不存在时,大肠杆菌中的Cascade基因会被StpA蛋白抑制。具有与H-NS相似的DNA结合偏好。在这里,我们显示StpA在含有抗λ间隔子的Δhns细胞中的过表达消除了对λvir感染的抵抗力,并减少了casA基因的转录。在缺乏hns和stpA基因的细胞中,casA基因的转录水平高于Δhns,与过表达LeuO的wt细胞相似。综上,这些结果表明,当H-NS不存在时,大肠杆菌中的Cascade基因会被StpA蛋白抑制。具有与H-NS相似的DNA结合偏好。在这里,我们显示StpA在含有抗λ间隔子的Δhns细胞中的过表达消除了对λvir感染的抵抗力,并减少了casA基因的转录。在缺乏hns和stpA基因的细胞中,casA基因的转录水平高于Δhns,与过表达LeuO的wt细胞相似。两者合计,这些结果表明当H-NS不存在时,大肠杆菌中的Cascade基因被StpA蛋白抑制。
更新日期:2020-04-28
中文翻译:

StpA抑制H-NS缺陷型大肠杆菌中的CRISPR-Cas免疫。
功能性CRISPR-Cas系统为许多细菌和大多数古细菌提供了针对入侵DNA元素的适应性免疫力。CRISPR阵列可存储先前感染的DNA片段,而cas基因产物可通过整合新的DNA片段并利用此信息识别和破坏入侵的DNA来提供免疫力。大肠杆菌包含CRISPR-Cas型IE系统,其中外源DNA靶点被Cascade识别,Cascade是由crRNA引导的复合物,包含5种蛋白质(CasA,CasB,CasC,CasD,CasE),并被Cas3降解。在大肠杆菌中,CRISPR-Cas型IE系统被组蛋白样核糖结构蛋白H-NS抑制。H-NS抑制可通过hns基因失活或升高水平的H-NS拮抗剂LeuO缓解,后者诱导cas基因的转录水平高于Δhns细胞。这表明Δhns细胞中的阻抑作用是不完全的,另外的阻遏物可能参与沉默。一种这样的候选物是H-NS旁系同源蛋白StpA,其具有与H-NS相似的DNA结合偏好。在这里,我们显示StpA在含有抗λ间隔子的Δhns细胞中的过表达消除了对λvir感染的抵抗力,并减少了casA基因的转录。在缺乏hns和stpA基因的细胞中,casA基因的转录水平高于Δhns,与过表达LeuO的wt细胞相似。综上,这些结果表明,当H-NS不存在时,大肠杆菌中的Cascade基因会被StpA蛋白抑制。具有与H-NS相似的DNA结合偏好。在这里,我们显示StpA在含有抗λ间隔子的Δhns细胞中的过表达消除了对λvir感染的抵抗力,并减少了casA基因的转录。在缺乏hns和stpA基因的细胞中,casA基因的转录水平高于Δhns,与过表达LeuO的wt细胞相似。综上,这些结果表明,当H-NS不存在时,大肠杆菌中的Cascade基因会被StpA蛋白抑制。具有与H-NS相似的DNA结合偏好。在这里,我们显示StpA在含有抗λ间隔子的Δhns细胞中的过表达消除了对λvir感染的抵抗力,并减少了casA基因的转录。在缺乏hns和stpA基因的细胞中,casA基因的转录水平高于Δhns,与过表达LeuO的wt细胞相似。两者合计,这些结果表明当H-NS不存在时,大肠杆菌中的Cascade基因被StpA蛋白抑制。



























 京公网安备 11010802027423号
京公网安备 11010802027423号