Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
D-loop Dynamics and Near-Atomic-Resolution Cryo-EM Structure of Phalloidin-Bound F-Actin.
Structure ( IF 4.4 ) Pub Date : 2020-04-28 , DOI: 10.1016/j.str.2020.04.004 Sanchaita Das 1 , Peng Ge 2 , Zeynep A Oztug Durer 1 , Elena E Grintsevich 1 , Z Hong Zhou 3 , Emil Reisler 4
Structure ( IF 4.4 ) Pub Date : 2020-04-28 , DOI: 10.1016/j.str.2020.04.004 Sanchaita Das 1 , Peng Ge 2 , Zeynep A Oztug Durer 1 , Elena E Grintsevich 1 , Z Hong Zhou 3 , Emil Reisler 4
Affiliation
|
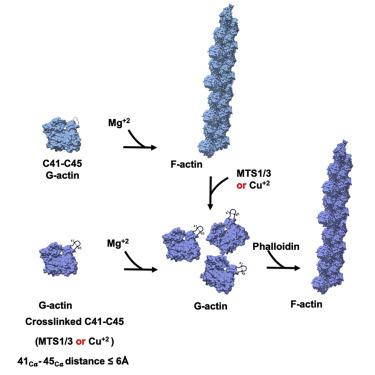
Detailed molecular information on G-actin assembly into filaments (F-actin), and their structure, dynamics, and interactions, is essential for understanding their cellular functions. Previous studies indicate that a flexible DNase I binding loop (D-loop, residues 40-50) plays a major role in actin's conformational dynamics. Phalloidin, a "gold standard" for actin filament staining, stabilizes them and affects the D-loop. Using disulfide crosslinking in yeast actin D-loop mutant Q41C/V45C, light-scattering measurements, and cryoelectron microscopy reconstructions, we probed the constraints of D-loop dynamics and its contribution to F-actin formation/stability. Our data support a model of residues 41-45 distances that facilitate G- to F-actin transition. We report also a 3.3-Å resolution structure of phalloidin-bound F-actin in the ADP-Pi-like (ADP-BeFx) state. This shows the phalloidin-binding site on F-actin and how the relative movement between its two protofilaments is restricted by it. Together, our results provide molecular details of F-actin structure and D-loop dynamics.
中文翻译:

Phalloidin结合的F-肌动蛋白的D环动力学和近原子分辨率的Cryo-EM结构。
有关G-肌动蛋白组装成细丝(F-肌动蛋白)及其结构,动力学和相互作用的详细分子信息对于理解其细胞功能至关重要。先前的研究表明,灵活的DNase I结合环(D环,残基40-50)在肌动蛋白的构象动力学中起主要作用。鬼笔环肽是肌动蛋白丝染色的“金标准”,可稳定它们并影响D环。使用酵母肌动蛋白D环突变体Q41C / V45C中的二硫键交联,光散射测量和低温电子显微镜重建,我们探究了D环动力学的局限性及其对F-肌动蛋白形成/稳定性的贡献。我们的数据支持残基41-45距离的模型,该模型有助于G-肌动蛋白过渡。我们还报告了3。在ADP-Pi-like(ADP-BeFx)状态下,与鬼笔环肽结合的F-肌动蛋白具有3-Å分辨率结构。这显示了F-肌动蛋白上的鬼笔环肽结合位点,以及它如何限制其两个原丝之间的相对运动。总之,我们的结果提供了F-肌动蛋白结构和D环动力学的分子细节。
更新日期:2020-04-28
中文翻译:

Phalloidin结合的F-肌动蛋白的D环动力学和近原子分辨率的Cryo-EM结构。
有关G-肌动蛋白组装成细丝(F-肌动蛋白)及其结构,动力学和相互作用的详细分子信息对于理解其细胞功能至关重要。先前的研究表明,灵活的DNase I结合环(D环,残基40-50)在肌动蛋白的构象动力学中起主要作用。鬼笔环肽是肌动蛋白丝染色的“金标准”,可稳定它们并影响D环。使用酵母肌动蛋白D环突变体Q41C / V45C中的二硫键交联,光散射测量和低温电子显微镜重建,我们探究了D环动力学的局限性及其对F-肌动蛋白形成/稳定性的贡献。我们的数据支持残基41-45距离的模型,该模型有助于G-肌动蛋白过渡。我们还报告了3。在ADP-Pi-like(ADP-BeFx)状态下,与鬼笔环肽结合的F-肌动蛋白具有3-Å分辨率结构。这显示了F-肌动蛋白上的鬼笔环肽结合位点,以及它如何限制其两个原丝之间的相对运动。总之,我们的结果提供了F-肌动蛋白结构和D环动力学的分子细节。











































 京公网安备 11010802027423号
京公网安备 11010802027423号