Structure ( IF 4.4 ) Pub Date : 2020-04-28 , DOI: 10.1016/j.str.2020.03.013 Dijun Du 1 , Arthur Neuberger 1 , Mona Wu Orr 2 , Catherine E Newman 1 , Pin-Chia Hsu 3 , Firdaus Samsudin 3 , Andrzej Szewczak-Harris 1 , Leana M Ramos 2 , Mekdes Debela 1 , Syma Khalid 3 , Gisela Storz 2 , Ben F Luisi 1
|
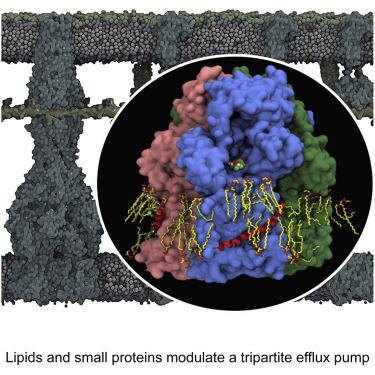
The small protein AcrZ in Escherichia coli interacts with the transmembrane portion of the multidrug efflux pump AcrB and increases resistance of the bacterium to a subset of the antibiotic substrates of that transporter. It is not clear how the physical association of the two proteins selectively changes activity of the pump for defined substrates. Here, we report cryo-EM structures of AcrB and the AcrBZ complex in lipid environments, and comparisons suggest that conformational changes occur in the drug-binding pocket as a result of AcrZ binding. Simulations indicate that cardiolipin preferentially interacts with the AcrBZ complex, due to increased contact surface, and we observe that chloramphenicol sensitivity of bacteria lacking AcrZ is exacerbated when combined with cardiolipin deficiency. Taken together, the data suggest that AcrZ and lipid cooperate to allosterically modulate AcrB activity. This mode of regulation by a small protein and lipid may occur for other membrane proteins.
中文翻译:

脂质环境中细菌 RND 转运蛋白与跨膜小蛋白的相互作用。
大肠杆菌中的小蛋白 AcrZ 与多药外排泵 AcrB 的跨膜部分相互作用,并增加细菌对该转运蛋白的抗生素底物子集的抵抗力。目前尚不清楚这两种蛋白质的物理结合如何选择性地改变特定底物的泵活性。在这里,我们报告了脂质环境中 AcrB 和 AcrBZ 复合物的冷冻电镜结构,比较表明,由于 AcrZ 结合,药物结合袋中发生了构象变化。模拟表明,由于接触表面增加,心磷脂优先与 AcrBZ 复合物相互作用,并且我们观察到,缺乏 AcrZ 的细菌在缺乏心磷脂时,其氯霉素敏感性会加剧。综上所述,数据表明 AcrZ 和脂质协同变构调节 AcrB 活性。这种由小蛋白质和脂质调节的模式可能发生在其他膜蛋白上。











































 京公网安备 11010802027423号
京公网安备 11010802027423号