Molecular and Biochemical Parasitology ( IF 1.4 ) Pub Date : 2020-04-28 , DOI: 10.1016/j.molbiopara.2020.111278 Andrea Vizcaíno-Castillo 1 , Juan Felipe Osorio-Méndez 2 , Javier R Ambrosio 3 , Roberto Hernández 1 , Ana María Cevallos 1
|
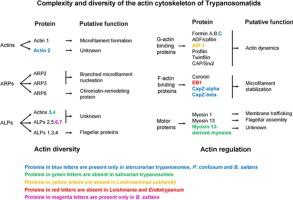
Trypanosomatids are a monophyletic group of parasitic flagellated protists belonging to the order Kinetoplastida. Their cytoskeleton is primarily made up of microtubules in which no actin microfilaments have been detected. Although all these parasites contain actin, it is widely thought that their actin cytoskeleton is reduced when compared to most eukaryotic organisms. However, there is increasing evidence that it is more complex than previously thought. As in other eukaryotic organisms, trypanosomatids encode for a conventional actin that is expected to form microfilament-like structures, and for members of three conserved actin-related proteins probably involved in microfilament nucleation (ARP2, ARP3) and in gene expression regulation (ARP6). In addition to these canonical proteins, also encode for an expanded set of actins and actin-like proteins that seem to be restricted to kinetoplastids. Analysis of their amino acid sequences demonstrated that, although very diverse in primary sequence when compared to actins of model organisms, modelling of their tertiary structure predicted the presence of the actin fold in all of them. Experimental characterization has been done for only a few of the trypanosomatid actins and actin-binding proteins. The most studied is the conventional actin of Leishmania donovani (LdAct), which unusually requires both ATP and Mg2+ for polymerization, unlike other conventional actins that do not require ATP. Additionally, polymerized LdAct tends to assemble in bundles rather than in single filaments. Regulation of actin polymerization depends on their interaction with actin-binding proteins. In trypanosomatids, there is a reduced but sufficient core of actin-binding proteins to promote microfilament nucleation, turnover and stabilization. There are also genes encoding for members of two families of myosin motor proteins, including one lineage-specific. Homologues to all identified actin-family proteins and actin-binding proteins of trypanosomatids are also present in Paratrypanosoma confusum (an early branching trypanosomatid) and in Bodo saltans (a closely related free-living organism belonging to the trypanosomatid sister order of Bodonida) suggesting they were all present in their common ancestor. Secondary losses of these genes may have occurred during speciation within the trypanosomatids, with salivarian trypanosomes having lost many of them and stercorarian trypanosomes retaining most.
中文翻译:

锥虫的肌动蛋白细胞骨架的复杂性和多样性。
锥虫科动物是属于鞭毛体科的单鞭毛寄生生物。它们的细胞骨架主要由未检测到肌动蛋白微丝的微管组成。尽管所有这些寄生虫均含有肌动蛋白,但与大多数真核生物相比,人们普遍认为其肌动蛋白的细胞骨架会减少。但是,越来越多的证据表明它比以前认为的要复杂。与其他真核生物一样,锥虫编码的是预期会形成微丝状结构的常规肌动蛋白,以及可能参与微丝成核(ARP2,ARP3)和基因表达调控(ARP6)的三种保守的肌动蛋白相关蛋白的成员。 。除了这些经典蛋白质之外,它还编码似乎仅限于运动质体的一系列肌动蛋白和肌动蛋白样蛋白。对它们的氨基酸序列的分析表明,尽管与模型生物的肌动蛋白相比,一级序列差异很大,但对其三级结构的建模预测了它们中肌动蛋白折叠的存在。仅对少数锥虫肌动蛋白和肌动蛋白结合蛋白进行了实验表征。研究最多的是传统的肌动蛋白 仅对少数锥虫肌动蛋白和肌动蛋白结合蛋白进行了实验表征。研究最多的是传统的肌动蛋白 仅对少数锥虫肌动蛋白和肌动蛋白结合蛋白进行了实验表征。研究最多的是传统的肌动蛋白利什曼原虫donovani(LdAct),与其他不需要ATP的常规肌动蛋白不同,它通常需要ATP和Mg 2+才能聚合。另外,聚合的LdAct倾向于成束组装,而不是单丝。肌动蛋白聚合的调节取决于它们与肌动蛋白结合蛋白的相互作用。在锥虫中,肌动蛋白结合蛋白的核心减少,但足以促进微丝成核,周转和稳定。也有编码肌球蛋白运动蛋白两个家族成员的基因,包括一种谱系特异性的。孔旁副孢子虫中也存在与所有已鉴定的锥虫的肌动蛋白家族蛋白和肌动蛋白结合蛋白同系物(早期分支的锥虫)和Bodo saltans(属于Bodonida锥虫的姐妹生活的密切相关的自由生物)表明它们都存在于其共同祖先中。这些基因的继发性丧失可能是在锥虫的物种形成过程中发生的,唾液中的锥虫失去了很多,而锥虫的锥虫保留了最多。









































 京公网安备 11010802027423号
京公网安备 11010802027423号