当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Origin of Solvent-Induced Polymorphism in Self-Assembly of Trimesic Acid Monolayers at Solid–Liquid Interfaces
Chemistry of Materials ( IF 7.2 ) Pub Date : 2020-04-28 , DOI: 10.1021/acs.chemmater.0c00827 Oliver Ochs 1, 2 , Manuela Hocke 1, 2 , Saskia Spitzer 1, 2 , Wolfgang M. Heckl 1, 2 , Natalia Martsinovich 3 , Markus Lackinger 1, 2
Chemistry of Materials ( IF 7.2 ) Pub Date : 2020-04-28 , DOI: 10.1021/acs.chemmater.0c00827 Oliver Ochs 1, 2 , Manuela Hocke 1, 2 , Saskia Spitzer 1, 2 , Wolfgang M. Heckl 1, 2 , Natalia Martsinovich 3 , Markus Lackinger 1, 2
Affiliation
|
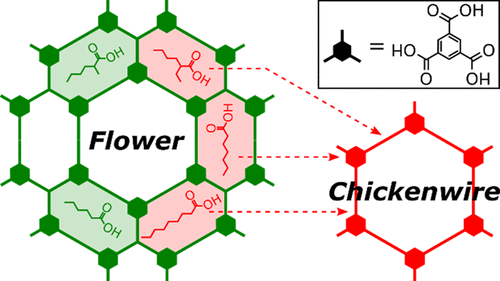
Encoding information in the chemical structure of tectons is the pivotal strategy in self-assembly for the realization of targeted supramolecular structures. However, frequently observed polymorphism in supramolecular monolayers provides experimental evidence for a decisive additional influence of environmental parameters, such as solute concentration or type of solvent, on structure selection. While concentration-induced polymorphism is comparatively well understood, the thermodynamic and molecular origins of solvent-induced polymorphism remain elusive. To shed light on this fundamental aspect of self-assembly, we explore the solvent-induced polymorphism of trimesic acid (TMA) monolayers on graphite as a prototypical example. Using the homologous series of fatty acids as solvents, TMA self-assembles into the anticipated chickenwire polymorph for longer chain fatty acids, whereas the more densely packed, but still porous flower polymorph emerges in shorter chain fatty acids. According to our initial working hypothesis, the origin of this solvent-induced polymorphism lies in the solvent dependence of the free energy gain. Utilizing an adapted Born–Haber cycle constructed from measured TMA sublimation and dissolution enthalpies as well as density functional theory-calculated monolayer binding energies, we quantitatively assessed the self-assembly thermodynamics of both polymorphs in hexanoic, heptanoic, and nonanoic acid. Yet, in contrast to the experimental findings, these results suggest superior thermodynamic stability of the chickenwire polymorph in all solvents. On the other hand, additional experiments comprising variable-temperature scanning tunneling microscopy corroborate that the flower polymorph is thermodynamically most stable in hexanoic acid. To resolve this apparent contradiction, we propose a thermodynamic stabilization of the flower polymorph in hexanoic acid through the stereochemically specific coadsorption of shape-matched solvent molecules in its unique smaller elongated pores. This alternative explanation gains further support from experiments with side-substituted hexanoic acid solvents. Combination of a quantitative thermodynamic analysis and studies with systematic variations of the solvent’s molecular structure holds great promise to enhance the understanding of thus far underexplored solvent effects.
中文翻译:

固液界面上三苯甲酸单层自组装中溶剂诱导的多态性的起源
在构造的化学结构中编码信息是实现目标超分子结构自组装的关键策略。然而,在超分子单层中经常观察到的多态性为环境参数(例如溶质浓度或溶剂类型)对结构选择的决定性附加影响提供了实验证据。尽管对浓度诱导的多态性的理解相对较好,但溶剂诱导的多态性的热力学和分子起源仍然难以捉摸。为了阐明自组装的这一基本方面,我们以石墨为例,探讨了溶剂诱导的均苯三酸(TMA)单层在石墨上的多态性。使用脂肪酸的同源系列作为溶剂,TMA会自组装成预期的长链脂肪酸多聚体,而更紧密堆积但仍是多孔的花状多聚体会出现在短链脂肪酸中。根据我们最初的工作假设,这种溶剂诱导的多态性的根源在于自由能增加的溶剂依赖性。利用通过测量的TMA升华和溶解焓以及密度泛函理论计算的单层结合能构建的适应的Born-Haber循环,我们定量评估了己酸,庚酸和壬酸中两种多晶型物的自组装热力学。然而,与实验结果相反,这些结果表明在所有溶剂中,鸡丝多晶型物具有优异的热力学稳定性。另一方面,包含可变温度扫描隧道显微镜的其他实验证实了该花多晶型物在己酸中在热力学上最稳定。为了解决这个明显的矛盾,我们提出了通过在形状匹配的溶剂分子在其独特的较小的细长孔中进行立体化学特异性共吸附,在己酸中实现花多晶型物的热力学稳定化。这种替代解释得到了侧取代己酸溶剂实验的进一步支持。将定量热力学分析与研究与溶剂分子结构的系统变化相结合,具有很大的希望,可以增进对迄今为止尚未充分探索的溶剂作用的理解。
更新日期:2020-06-23
中文翻译:

固液界面上三苯甲酸单层自组装中溶剂诱导的多态性的起源
在构造的化学结构中编码信息是实现目标超分子结构自组装的关键策略。然而,在超分子单层中经常观察到的多态性为环境参数(例如溶质浓度或溶剂类型)对结构选择的决定性附加影响提供了实验证据。尽管对浓度诱导的多态性的理解相对较好,但溶剂诱导的多态性的热力学和分子起源仍然难以捉摸。为了阐明自组装的这一基本方面,我们以石墨为例,探讨了溶剂诱导的均苯三酸(TMA)单层在石墨上的多态性。使用脂肪酸的同源系列作为溶剂,TMA会自组装成预期的长链脂肪酸多聚体,而更紧密堆积但仍是多孔的花状多聚体会出现在短链脂肪酸中。根据我们最初的工作假设,这种溶剂诱导的多态性的根源在于自由能增加的溶剂依赖性。利用通过测量的TMA升华和溶解焓以及密度泛函理论计算的单层结合能构建的适应的Born-Haber循环,我们定量评估了己酸,庚酸和壬酸中两种多晶型物的自组装热力学。然而,与实验结果相反,这些结果表明在所有溶剂中,鸡丝多晶型物具有优异的热力学稳定性。另一方面,包含可变温度扫描隧道显微镜的其他实验证实了该花多晶型物在己酸中在热力学上最稳定。为了解决这个明显的矛盾,我们提出了通过在形状匹配的溶剂分子在其独特的较小的细长孔中进行立体化学特异性共吸附,在己酸中实现花多晶型物的热力学稳定化。这种替代解释得到了侧取代己酸溶剂实验的进一步支持。将定量热力学分析与研究与溶剂分子结构的系统变化相结合,具有很大的希望,可以增进对迄今为止尚未充分探索的溶剂作用的理解。











































 京公网安备 11010802027423号
京公网安备 11010802027423号