Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Physico-chemical properties of two point mutants of small heat shock protein HspB6 (Hsp20) with abrogated cardioprotection.
Biochimie ( IF 3.3 ) Pub Date : 2020-04-27 , DOI: 10.1016/j.biochi.2020.04.021 Vladislav M Shatov 1 , Nikolai B Gusev 1
Biochimie ( IF 3.3 ) Pub Date : 2020-04-27 , DOI: 10.1016/j.biochi.2020.04.021 Vladislav M Shatov 1 , Nikolai B Gusev 1
Affiliation
|
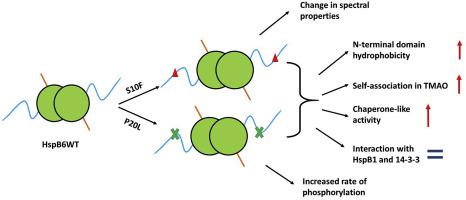
Physico-chemical properties of HspB6 S10F and P20L mutants with abrogated cardioprotective activity and associated with different forms of cardiomyopathy were analyzed. Under normal conditions both the wild-type HspB6 and its mutants formed small size oligomers (dimers) with apparent molecular weight of 50-60 kDa. Under crowding conditions (0.5 M trimethylamine N-oxide, TMAO) the wild-type HspB6 remained predominantly dimeric or formed small molecular weight complexes, whereas both mutants tended to form high molecular weight complexes. Catalytic subunit of cAMP-dependent protein kinase phosphorylated the wild-type HspB6 and its S10F mutant with comparable rate. The rate of P20L mutant phosphorylation was higher than that of the wild-type HspB6. S10F and P20L mutations did not affect interaction of phosphorylated HspB6 with universal adapter proteins 14-3-3. The wild-type HspB6 was resistant to heat-induced denaturation and aggregation, whereas both its mutants were denatured and started to aggregate at temperature much lower than its wild-type counterpart. Titration with fluorescent probe bis-ANS was accompanied by larger increase of fluorescence in the case of both mutants than in the case of the wild-type HspB6. Both mutants possessed higher chaperone-like activity than the wild-type protein. It is concluded that both S10F and P20L mutations are accompanied by increase of hydrophobicity of the very N-terminal region of HspB6 leading to increased aggregation at elevated temperature, formation of large complexes under crowding conditions and increased chaperone-like activity measured in vitro. Increased hydrophobicity and self-association can affect substrate specificity and interaction with certain target proteins thus leading to decrease or complete abrogation of cardioprotective activity.
中文翻译:

小型热休克蛋白HspB6(Hsp20)的两个点突变体的心脏化学特性,具有被取消的心脏保护作用。
分析了具有取消的心脏保护活性并与不同形式的心肌病相关的HspB6 S10F和P20L突变体的理化特性。在正常条件下,野生型HspB6及其突变体均形成表观分子量为50-60 kDa的小尺寸寡聚体(二聚体)。在拥挤条件下(0.5 M三甲胺N-氧化物,TMAO),野生型HspB6主要保持二聚体或形成小分子量复合物,而两个突变体均倾向于形成高分子量复合物。cAMP依赖性蛋白激酶的催化亚基以相当的速率磷酸化了野生型HspB6及其S10F突变体。P20L突变体的磷酸化率高于野生型HspB6。S10F和P20L突变不影响磷酸化的HspB6与通用衔接蛋白14-3-3的相互作用。野生型HspB6对热诱导的变性和聚集具有抗性,而其两个突变体均变性并在远低于其野生型对应物的温度下开始聚集。与野生型HspB6相比,在两个突变体中用荧光探针bis-ANS滴定伴随的荧光增加更大。两种突变体都具有比野生型蛋白更高的伴侣样活性。结论是S10F和P20L突变都伴随着HspB6 N末端区域疏水性的增加,导致在高温下聚集增加,拥挤条件下形成大分子复合物,体外测得的类伴侣分子活性增加。增加的疏水性和自缔合可影响底物特异性和与某些靶蛋白的相互作用,从而导致心脏保护活性降低或完全丧失。
更新日期:2020-04-27
中文翻译:

小型热休克蛋白HspB6(Hsp20)的两个点突变体的心脏化学特性,具有被取消的心脏保护作用。
分析了具有取消的心脏保护活性并与不同形式的心肌病相关的HspB6 S10F和P20L突变体的理化特性。在正常条件下,野生型HspB6及其突变体均形成表观分子量为50-60 kDa的小尺寸寡聚体(二聚体)。在拥挤条件下(0.5 M三甲胺N-氧化物,TMAO),野生型HspB6主要保持二聚体或形成小分子量复合物,而两个突变体均倾向于形成高分子量复合物。cAMP依赖性蛋白激酶的催化亚基以相当的速率磷酸化了野生型HspB6及其S10F突变体。P20L突变体的磷酸化率高于野生型HspB6。S10F和P20L突变不影响磷酸化的HspB6与通用衔接蛋白14-3-3的相互作用。野生型HspB6对热诱导的变性和聚集具有抗性,而其两个突变体均变性并在远低于其野生型对应物的温度下开始聚集。与野生型HspB6相比,在两个突变体中用荧光探针bis-ANS滴定伴随的荧光增加更大。两种突变体都具有比野生型蛋白更高的伴侣样活性。结论是S10F和P20L突变都伴随着HspB6 N末端区域疏水性的增加,导致在高温下聚集增加,拥挤条件下形成大分子复合物,体外测得的类伴侣分子活性增加。增加的疏水性和自缔合可影响底物特异性和与某些靶蛋白的相互作用,从而导致心脏保护活性降低或完全丧失。











































 京公网安备 11010802027423号
京公网安备 11010802027423号