当前位置:
X-MOL 学术
›
J. Struct. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure of the enterovirus D68 RNA-dependent RNA polymerase in complex with NADPH implicates an inhibitor binding site in the RNA template tunnel.
Journal of Structural Biology ( IF 3.0 ) Pub Date : 2020-04-27 , DOI: 10.1016/j.jsb.2020.107510 Li Li 1 , Meilin Wang 1 , Yiping Chen 1 , Tingting Hu 1 , Yan Yang 1 , Yang Zhang 1 , Gang Bi 1 , Wei Wang 2 , Enmei Liu 3 , Junhong Han 1 , Tao Lu 4 , Dan Su 5
Journal of Structural Biology ( IF 3.0 ) Pub Date : 2020-04-27 , DOI: 10.1016/j.jsb.2020.107510 Li Li 1 , Meilin Wang 1 , Yiping Chen 1 , Tingting Hu 1 , Yan Yang 1 , Yang Zhang 1 , Gang Bi 1 , Wei Wang 2 , Enmei Liu 3 , Junhong Han 1 , Tao Lu 4 , Dan Su 5
Affiliation
|
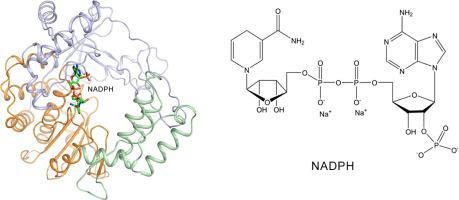
Enterovirus D68 (EV-D68) is an emerging viral pathogen belonging to the Enterovirus genus of the Picornaviridae family, which is a serious threat to human health and has resulted in significant economic losses. The EV-D68 genome encodes an RNA-dependent RNA polymerase (RdRp) 3Dpol, which is central for viral genome replication and considered as a promising target for specific antiviral therapeutics. In this study, we report the crystal structures of human EV-D68 RdRp in the apo state and in complex with the inhibitor NADPH, which was selected by using a structure-based virtual screening approach. The EV-D68-RdRp-NADPH complex is the first RdRp-inhibitor structure identified in the species Enterovirus D. The inhibitor NADPH occupies the RNA template binding channel of EV-D68 RdRp with a novel binding pocket. Additionally, residues involved in the NADPH binding pocket of EV-D68 RdRp are highly conserved in RdRps of enteroviruses. Therefore, the enzyme activity of three RdRps from EV-D68, poliovirus, and enterovirus A71 is shown to decrease when titrated with NADPH separately in vitro. Furthermore, we identified that NADPH plays a pivotal role as an RdRp inhibitor instead of a chain terminator during restriction of RNA-dependent RNA replication. In the future, derivatives of NADPH may pave the way for novel inhibitors of RdRp through compound modification, providing potential antiviral agents for treating enteroviral infection and related diseases.
中文翻译:

与 NADPH 复合的肠道病毒 D68 RNA 依赖性 RNA 聚合酶的结构暗示 RNA 模板隧道中的抑制剂结合位点。
肠道病毒D68(EV-D68)是一种新兴的病毒病原体,属于小核糖核酸病毒科肠道病毒属,严重威胁人类健康并造成重大经济损失。EV-D68 基因组编码一种依赖于 RNA 的 RNA 聚合酶 (RdRp) 3Dpol,它是病毒基因组复制的核心,被认为是特定抗病毒治疗的有希望的靶点。在这项研究中,我们报告了人类 EV-D68 RdRp 处于载脂蛋白状态并与抑制剂 NADPH 复合的晶体结构,该抑制剂是通过使用基于结构的虚拟筛选方法选择的。EV-D68-RdRp-NADPH 复合物是第一个在肠道病毒 D 种中发现的 RdRp 抑制剂结构。抑制剂 NADPH 占据了 EV-D68 RdRp 的 RNA 模板结合通道,并带有一个新的结合口袋。此外,EV-D68 RdRp 的 NADPH 结合口袋中涉及的残基在肠道病毒的 RdRps 中高度保守。因此,在体外分别用 NADPH 滴定时,来自 EV-D68、脊髓灰质炎病毒和肠道病毒 A71 的三种 RdRps 的酶活性显示降低。此外,我们发现 NADPH 在限制依赖于 RNA 的 RNA 复制期间作为 RdRp 抑制剂而不是链终止子发挥着关键作用。未来,NADPH的衍生物可能通过化合物修饰为新型RdRp抑制剂铺平道路,为治疗肠道病毒感染及相关疾病提供潜在的抗病毒药物。在体外分别用 NADPH 滴定时,肠道病毒 A71 显示减少。此外,我们发现 NADPH 在限制依赖于 RNA 的 RNA 复制期间作为 RdRp 抑制剂而不是链终止子发挥着关键作用。未来,NADPH的衍生物可能通过化合物修饰为新型RdRp抑制剂铺平道路,为治疗肠道病毒感染及相关疾病提供潜在的抗病毒药物。在体外分别用 NADPH 滴定时,肠道病毒 A71 显示减少。此外,我们发现 NADPH 在限制依赖于 RNA 的 RNA 复制期间作为 RdRp 抑制剂而不是链终止子发挥着关键作用。未来,NADPH的衍生物可能通过化合物修饰为新型RdRp抑制剂铺平道路,为治疗肠道病毒感染及相关疾病提供潜在的抗病毒药物。
更新日期:2020-04-27
中文翻译:

与 NADPH 复合的肠道病毒 D68 RNA 依赖性 RNA 聚合酶的结构暗示 RNA 模板隧道中的抑制剂结合位点。
肠道病毒D68(EV-D68)是一种新兴的病毒病原体,属于小核糖核酸病毒科肠道病毒属,严重威胁人类健康并造成重大经济损失。EV-D68 基因组编码一种依赖于 RNA 的 RNA 聚合酶 (RdRp) 3Dpol,它是病毒基因组复制的核心,被认为是特定抗病毒治疗的有希望的靶点。在这项研究中,我们报告了人类 EV-D68 RdRp 处于载脂蛋白状态并与抑制剂 NADPH 复合的晶体结构,该抑制剂是通过使用基于结构的虚拟筛选方法选择的。EV-D68-RdRp-NADPH 复合物是第一个在肠道病毒 D 种中发现的 RdRp 抑制剂结构。抑制剂 NADPH 占据了 EV-D68 RdRp 的 RNA 模板结合通道,并带有一个新的结合口袋。此外,EV-D68 RdRp 的 NADPH 结合口袋中涉及的残基在肠道病毒的 RdRps 中高度保守。因此,在体外分别用 NADPH 滴定时,来自 EV-D68、脊髓灰质炎病毒和肠道病毒 A71 的三种 RdRps 的酶活性显示降低。此外,我们发现 NADPH 在限制依赖于 RNA 的 RNA 复制期间作为 RdRp 抑制剂而不是链终止子发挥着关键作用。未来,NADPH的衍生物可能通过化合物修饰为新型RdRp抑制剂铺平道路,为治疗肠道病毒感染及相关疾病提供潜在的抗病毒药物。在体外分别用 NADPH 滴定时,肠道病毒 A71 显示减少。此外,我们发现 NADPH 在限制依赖于 RNA 的 RNA 复制期间作为 RdRp 抑制剂而不是链终止子发挥着关键作用。未来,NADPH的衍生物可能通过化合物修饰为新型RdRp抑制剂铺平道路,为治疗肠道病毒感染及相关疾病提供潜在的抗病毒药物。在体外分别用 NADPH 滴定时,肠道病毒 A71 显示减少。此外,我们发现 NADPH 在限制依赖于 RNA 的 RNA 复制期间作为 RdRp 抑制剂而不是链终止子发挥着关键作用。未来,NADPH的衍生物可能通过化合物修饰为新型RdRp抑制剂铺平道路,为治疗肠道病毒感染及相关疾病提供潜在的抗病毒药物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号