Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unveiling the Axial Hydroxyl Ligand on Fe-N4-C Electrocatalysts and Its Impact on the pH-Dependent Oxygen Reduction Activities and Poisoning Kinetics.
Advanced Science ( IF 14.3 ) Pub Date : 2020-04-27 , DOI: 10.1002/advs.202000176 Xin Yang 1, 2 , Dongsheng Xia 1 , Yongqiang Kang 1 , Hongda Du 1 , Feiyu Kang 1 , Lin Gan 1 , Jia Li 1, 2
Advanced Science ( IF 14.3 ) Pub Date : 2020-04-27 , DOI: 10.1002/advs.202000176 Xin Yang 1, 2 , Dongsheng Xia 1 , Yongqiang Kang 1 , Hongda Du 1 , Feiyu Kang 1 , Lin Gan 1 , Jia Li 1, 2
Affiliation
|
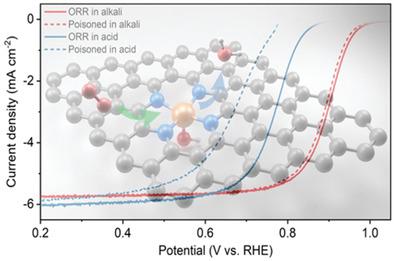
FeNC materials have shown a promising nonprecious oxygen reduction reaction (ORR) electrocatalyst yet their active site structure remains elusive. Several previous works suggest the existence of a mysterious axial ligand on the Fe center, which, however, is still unclarified. In this study, the mysterious axial ligand is identified as a hydroxyl ligand on the Fe centers and selectively promotes the ORR activities depending on different FeN4C configurations, on which the adsorption free energy of the hydroxyl ligand also differs greatly. The selective formation of hydroxyl ligand on specific FeNC configurations can resolve contradictories between previous theoretical and experimental results regarding the ORR activities and associated active configurations of FeNC catalysts. It also explains the pH‐dependent ORR activities and, moreover, a previously unreported pH‐dependent poisoning kinetics of the FeNC catalysts.
中文翻译:

揭示 Fe-N4-C 电催化剂上的轴向羟基配体及其对 pH 依赖性氧还原活性和中毒动力学的影响。
FeNC材料已显示出一种有前途的非贵金属氧还原反应(ORR)电催化剂,但其活性位点结构仍然难以捉摸。之前的几项工作表明,Fe 中心存在一个神秘的轴向配体,然而,这一点仍不清楚。在这项研究中,神秘的轴向配体被确定为Fe中心上的羟基配体,并根据不同的FeN 4C构型选择性地促进ORR活性,其中羟基配体的吸附自由能也有很大差异。在特定的FeNC构型上选择性形成羟基配体可以解决先前关于FeNC催化剂的ORR活性和相关活性构型的理论和实验结果之间的矛盾。它还解释了 pH 依赖性 ORR 活性,以及之前未报道的 Fe N C 催化剂的 pH 依赖性中毒动力学。
更新日期:2020-06-24
中文翻译:

揭示 Fe-N4-C 电催化剂上的轴向羟基配体及其对 pH 依赖性氧还原活性和中毒动力学的影响。
FeNC材料已显示出一种有前途的非贵金属氧还原反应(ORR)电催化剂,但其活性位点结构仍然难以捉摸。之前的几项工作表明,Fe 中心存在一个神秘的轴向配体,然而,这一点仍不清楚。在这项研究中,神秘的轴向配体被确定为Fe中心上的羟基配体,并根据不同的FeN 4C构型选择性地促进ORR活性,其中羟基配体的吸附自由能也有很大差异。在特定的FeNC构型上选择性形成羟基配体可以解决先前关于FeNC催化剂的ORR活性和相关活性构型的理论和实验结果之间的矛盾。它还解释了 pH 依赖性 ORR 活性,以及之前未报道的 Fe N C 催化剂的 pH 依赖性中毒动力学。











































 京公网安备 11010802027423号
京公网安备 11010802027423号