Computational and Theoretical Chemistry ( IF 3.0 ) Pub Date : 2020-04-27 , DOI: 10.1016/j.comptc.2020.112834 Friedrich Grein
|
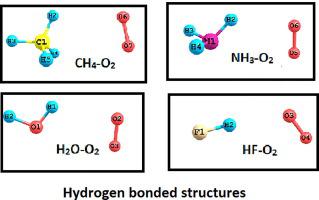
Using coupled cluster singles, doubles and perturbative triples CCSD(T) methods with augmented correlation consistent basis sets up to the 5Z level, dissociation energies and structural parameters were obtained for CH4-O2, NH3-O2, H2O-O2 and HF-O2 triplet complexes. Most stable for CH4-O2 is a structure with three hydrogens facing O2 in T-shape (dissociation energy De=168 cm-1), for NH3-O2 a structure with two hydrogens facing O2 in X shape (De=196 cm-1), and for H2O-O2 (De=222 cm-1) and HF-O2 (De=301 cm-1) a hydrogen bonded structure. Energies, geometries, vibrational frequencies, infrared intensities and dipole/quadrupole moments of the four complexes were compared. While such properties change gradually from CH4-O2 to H2O-O2, they are much more pronounced for HF-O2. The transition from van der Waals to hydrogen bonding was followed. There are significant changes in the O2 frequencies for hydrogen-bonded structures. Due to increased vibrational intensities such complexes may contribute to the greenhouse effect.
中文翻译:

CH 4 -O 2,NH 3 -O 2,H 2 O-O 2和HF-O 2三重态络合物。从头算起研究和比较。从范德华斯到氢键
使用耦合的单,双和扰动三重耦合CCSD(T)方法,将相关一致性基础设置提高到5Z级,获得CH 4 -O 2,NH 3 -O 2,H 2 O-O 2的解离能和结构参数和HF-O 2三重态络合物。对于CH 4 -O 2最稳定的是三个氢以T形面向O 2的结构(解离能D e = 168 cm -1),对于NH 3 -O 2是两个氢的X形面向O 2的结构。 (De = 196 cm -1),对于H 2 O-O 2(D e = 222 cm -1)和HF-O 2(D e = 301 cm -1),为氢键结构。比较了这四个配合物的能量,几何形状,振动频率,红外强度和偶极/四极矩。尽管这种性质从CH 4 -O 2逐渐变为H 2 O-O 2,但对于HF-O 2则更为明显。随后进行了从范德华斯到氢键的转变。O 2有重大变化氢键结构的频率。由于增加的振动强度,此类配合物可能有助于温室效应。











































 京公网安备 11010802027423号
京公网安备 11010802027423号