当前位置:
X-MOL 学术
›
Bioorg. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sulfonamide-based 4-anilinoquinoline derivatives as novel dual Aurora kinase (AURKA/B) inhibitors: Synthesis, biological evaluation and in silico insights.
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-04-25 , DOI: 10.1016/j.bmc.2020.115525 Mohammad M Al-Sanea 1 , Ahmed Elkamhawy 2 , Sora Paik 3 , Kyeong Lee 4 , Ahmed M El Kerdawy 5 , Bukhari Syed Nasir Abbas 1 , Eun Joo Roh 6 , Wagdy M Eldehna 7 , Heba A H Elshemy 8 , Rania B Bakr 9 , Ibrahim Ali Farahat 10 , Abdulaziz I Alzarea 11 , Sami I Alzarea 12 , Khalid S Alharbi 12 , Mohamed A Abdelgawad 9
Bioorganic & Medicinal Chemistry ( IF 3.3 ) Pub Date : 2020-04-25 , DOI: 10.1016/j.bmc.2020.115525 Mohammad M Al-Sanea 1 , Ahmed Elkamhawy 2 , Sora Paik 3 , Kyeong Lee 4 , Ahmed M El Kerdawy 5 , Bukhari Syed Nasir Abbas 1 , Eun Joo Roh 6 , Wagdy M Eldehna 7 , Heba A H Elshemy 8 , Rania B Bakr 9 , Ibrahim Ali Farahat 10 , Abdulaziz I Alzarea 11 , Sami I Alzarea 12 , Khalid S Alharbi 12 , Mohamed A Abdelgawad 9
Affiliation
|
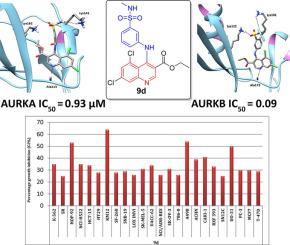
Aurora kinases (AURKs) were identified as promising druggable targets for targeted cancer therapy. Aiming at the development of novel chemotype of dual AURKA/B inhibitors, herein we report the design and synthesis of three series of 4-anilinoquinoline derivatives bearing a sulfonamide moiety (5a-d, 9a-d and 11a-d). The % inhibition of AURKA/B was determined for all target quinolines, then compounds showed more than 50% inhibition on either of the enzymes, were evaluated further for their IC50 on the corresponding enzyme. In particular, compound 9d displayed potent AURKA/B inhibitory activities with IC50 of 0.93 and 0.09 µM, respectively. Also, 9d emerged as the most efficient anti-proliferative analogue in the US-NCI anticancer assay toward the NCI 60 cell lines panel, with broad spectrum activity against different cell lines from diverse cancer subpanels. Docking studies, confirmed that, the sulfonamide SO2 oxygen was involved in a hydrogen bond with Lys162 and Lys122 in AURKA and AURKB, respectively, whereas, the sulfonamide NH could catch hydrogen bond interaction with the surrounding amino acid residues Lys141, Glu260, and Asn261 in AURKA and Lys101, Glu177, and Asp234 in AURKB. Furthermore, N1 nitrogen of the quinoline scaffold formed an essential hydrogen bond with the hinge region key amino acids Ala213 and Ala173 in AURKA and AURKB, respectively.
中文翻译:

基于磺酰胺的4-苯胺基喹啉衍生物作为新型双重Aurora激酶(AURKA / B)抑制剂:合成,生物学评估和计算机分析。
极光激酶(AURK)被确定为靶向癌症治疗的有希望的药物靶标。针对双AURKA / B抑制剂的新型化学型的发展,在此我们报道了设计和合成三个系列的带有磺酰胺基团的4-苯胺基喹啉衍生物(5a-d,9a-d和11a-d)。确定所有目标喹啉的AURKA / B抑制%,然后对任一酶显示50%以上的抑制率,然后进一步评估其对相应酶的IC50。特别是化合物9d表现出强大的AURKA / B抑制活性,IC50分别为0.93和0.09 µM。同样,在US-NCI抗癌试验中,针对NCI 60细胞系的9d成为最有效的抗增殖类似物,具有针对来自不同癌症子面板的不同细胞系的广谱活性。对接研究证实,磺酰胺SO2的氧分别与AURKA和AURKB中的Lys162和Lys122形成氢键,而磺酰胺NH可以捕获与周围氨基酸残基Lys141,Glu260和Asn261的氢键相互作用。 AURKA中的AURKA和Lys101,Glu177和Asp234。此外,喹啉骨架的N1氮分别与AURKA和AURKB中的铰链区关键氨基酸Ala213和Ala173形成必要的氢键。磺酰胺NH可以捕获与AURKA中的周围氨基酸残基Lys141,Glu260和Asn261和AURKB中的Lys101,Glu177和Asp234的氢键相互作用。此外,喹啉骨架的N1氮分别与AURKA和AURKB中的铰链区关键氨基酸Ala213和Ala173形成必要的氢键。磺酰胺NH可以捕获与AURKA中的周围氨基酸残基Lys141,Glu260和Asn261和AURKB中的Lys101,Glu177和Asp234的氢键相互作用。此外,喹啉骨架的N1氮分别与AURKA和AURKB中的铰链区关键氨基酸Ala213和Ala173形成必要的氢键。
更新日期:2020-04-25
中文翻译:

基于磺酰胺的4-苯胺基喹啉衍生物作为新型双重Aurora激酶(AURKA / B)抑制剂:合成,生物学评估和计算机分析。
极光激酶(AURK)被确定为靶向癌症治疗的有希望的药物靶标。针对双AURKA / B抑制剂的新型化学型的发展,在此我们报道了设计和合成三个系列的带有磺酰胺基团的4-苯胺基喹啉衍生物(5a-d,9a-d和11a-d)。确定所有目标喹啉的AURKA / B抑制%,然后对任一酶显示50%以上的抑制率,然后进一步评估其对相应酶的IC50。特别是化合物9d表现出强大的AURKA / B抑制活性,IC50分别为0.93和0.09 µM。同样,在US-NCI抗癌试验中,针对NCI 60细胞系的9d成为最有效的抗增殖类似物,具有针对来自不同癌症子面板的不同细胞系的广谱活性。对接研究证实,磺酰胺SO2的氧分别与AURKA和AURKB中的Lys162和Lys122形成氢键,而磺酰胺NH可以捕获与周围氨基酸残基Lys141,Glu260和Asn261的氢键相互作用。 AURKA中的AURKA和Lys101,Glu177和Asp234。此外,喹啉骨架的N1氮分别与AURKA和AURKB中的铰链区关键氨基酸Ala213和Ala173形成必要的氢键。磺酰胺NH可以捕获与AURKA中的周围氨基酸残基Lys141,Glu260和Asn261和AURKB中的Lys101,Glu177和Asp234的氢键相互作用。此外,喹啉骨架的N1氮分别与AURKA和AURKB中的铰链区关键氨基酸Ala213和Ala173形成必要的氢键。磺酰胺NH可以捕获与AURKA中的周围氨基酸残基Lys141,Glu260和Asn261和AURKB中的Lys101,Glu177和Asp234的氢键相互作用。此外,喹啉骨架的N1氮分别与AURKA和AURKB中的铰链区关键氨基酸Ala213和Ala173形成必要的氢键。











































 京公网安备 11010802027423号
京公网安备 11010802027423号