当前位置:
X-MOL 学术
›
Appl. Geochem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Oxygen atom release during selenium oxyanion adsorption on goethite and hematite
Applied Geochemistry ( IF 3.1 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.apgeochem.2020.104605 Pu Yue , Ning Chen , Derek Peak , Nefeli Maria Bompoti , Maria Chrysochoou , Annalisa Onnis-Hayden , Philip Larese-Casanova
Applied Geochemistry ( IF 3.1 ) Pub Date : 2020-06-01 , DOI: 10.1016/j.apgeochem.2020.104605 Pu Yue , Ning Chen , Derek Peak , Nefeli Maria Bompoti , Maria Chrysochoou , Annalisa Onnis-Hayden , Philip Larese-Casanova
|
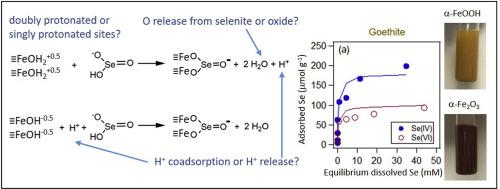
Abstract Adsorption of selenium oxyanions (selenite and selenate) on soil minerals can mitigate Se ecotoxicity effects by removing Se from water resources. Se oxyanion surface complexation on Fe oxides have been investigated for decades using spectroscopic and surface complex modeling. Here, insights on the Fe-O-Se bonding process on goethite and hematite were gained via integrating our observed batch Se adsorption isotherms, H+ coadsorption with Se, X-ray absorption spectroscopy (XAS)-derived surface complex geometry, surface complexation modeling, and 18O tracing across oxide and aqueous phases Adsorption extents of selenite and selenate on goethite and hematite decreased as pH increased from 3.0 to 7.0 and follows a corresponding decrease in positive zeta potential which offers less electrostatic attraction. Surface complexation geometry was determined with XAS results that indicated the formation of inner-sphere binuclear bidentate selenite complexes and outer-sphere selenate complexes on both oxides within the range of experimental conditions. These surface complexes were sufficient to model Se adsorption isotherms and H+ coadsorption on both oxides at pH 3.0, 5.0, and 7.0 using the Charge Distribution Multi-site Complexation (CD-MUSIC) model. Inner-sphere bidentate selenite complex formation requires release of two O, and by tracing 18O release from 18O-enriched oxides to isotopically normal water, it was found that O release from the oxide surface accounted for 22% and 7% of total adsorption-induced O release for goethite and hematite, respectively, but only at pH 3.0. The remaining O release is assumed to be from adsorbed selenite. Selenate at pH 3.0 also induced a slight increase in 18O enrichment in bulk solution, which is unexpected for selenate's outer-sphere configuration that should produce no O ligand exchange, but instead highlights some spontaneous adsorption-induced oxide O release.
中文翻译:

针铁矿和赤铁矿吸附硒氧阴离子过程中氧原子的释放
摘要 硒氧阴离子(亚硒酸盐和硒酸盐)在土壤矿物质上的吸附可以通过去除水资源中的硒来减轻硒的生态毒性效应。几十年来,人们已经使用光谱和表面复合模型研究了 Fe 氧化物上的 Se 氧阴离子表面复合。在这里,通过整合我们观察到的批量 Se 吸附等温线、H+ 与 Se 的共吸附、X 射线吸收光谱 (XAS) 衍生的表面复合几何形状、表面复合建模,获得了对针铁矿和赤铁矿 Fe-O-Se 键合过程的见解,和 18O 跨越氧化物和水相示踪 随着 pH 从 3.0 增加到 7.0,亚硒酸盐和硒酸盐在针铁矿和赤铁矿上的吸附程度降低,并且正 zeta 电位相应降低,从而提供较小的静电吸引力。表面络合几何形状由 XAS 结果确定,该结果表明在实验条件范围内,两种氧化物上形成了内球双核双齿亚硒酸盐复合物和外球硒酸盐复合物。这些表面复合物足以使用电荷分布多位点复合 (CD-MUSIC) 模型模拟 pH 3.0、5.0 和 7.0 下两种氧化物上的 Se 吸附等温线和 H+ 共吸附。球内双齿亚硒酸盐复合物的形成需要释放两个 O,通过追踪 18O 从富含 18O 的氧化物释放到同位素正常水,发现从氧化物表面释放的 O 占总吸附诱导的 22% 和 7% O 分别释放针铁矿和赤铁矿,但仅在 pH 3.0 时。假设剩余的 O 释放来自吸附的亚硒酸盐。
更新日期:2020-06-01
中文翻译:

针铁矿和赤铁矿吸附硒氧阴离子过程中氧原子的释放
摘要 硒氧阴离子(亚硒酸盐和硒酸盐)在土壤矿物质上的吸附可以通过去除水资源中的硒来减轻硒的生态毒性效应。几十年来,人们已经使用光谱和表面复合模型研究了 Fe 氧化物上的 Se 氧阴离子表面复合。在这里,通过整合我们观察到的批量 Se 吸附等温线、H+ 与 Se 的共吸附、X 射线吸收光谱 (XAS) 衍生的表面复合几何形状、表面复合建模,获得了对针铁矿和赤铁矿 Fe-O-Se 键合过程的见解,和 18O 跨越氧化物和水相示踪 随着 pH 从 3.0 增加到 7.0,亚硒酸盐和硒酸盐在针铁矿和赤铁矿上的吸附程度降低,并且正 zeta 电位相应降低,从而提供较小的静电吸引力。表面络合几何形状由 XAS 结果确定,该结果表明在实验条件范围内,两种氧化物上形成了内球双核双齿亚硒酸盐复合物和外球硒酸盐复合物。这些表面复合物足以使用电荷分布多位点复合 (CD-MUSIC) 模型模拟 pH 3.0、5.0 和 7.0 下两种氧化物上的 Se 吸附等温线和 H+ 共吸附。球内双齿亚硒酸盐复合物的形成需要释放两个 O,通过追踪 18O 从富含 18O 的氧化物释放到同位素正常水,发现从氧化物表面释放的 O 占总吸附诱导的 22% 和 7% O 分别释放针铁矿和赤铁矿,但仅在 pH 3.0 时。假设剩余的 O 释放来自吸附的亚硒酸盐。











































 京公网安备 11010802027423号
京公网安备 11010802027423号