当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reaction Pathway of the Hydrothermal Synthesis of AgCuO2 from In Situ Time-Resolved X-ray Diffraction
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2020-04-27 , DOI: 10.1021/acs.cgd.9b01516 Hongjun Liu 1 , Ola G. Grendal 2 , Susanne Linn Skjærvø 2 , Antoine R. M. Dalod 2 , Wouter van Beek 3 , Abderrahime Sekkat 1 , Mari-Ann Einarsrud 2 , David Muñoz-Rojas 1
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2020-04-27 , DOI: 10.1021/acs.cgd.9b01516 Hongjun Liu 1 , Ola G. Grendal 2 , Susanne Linn Skjærvø 2 , Antoine R. M. Dalod 2 , Wouter van Beek 3 , Abderrahime Sekkat 1 , Mari-Ann Einarsrud 2 , David Muñoz-Rojas 1
Affiliation
|
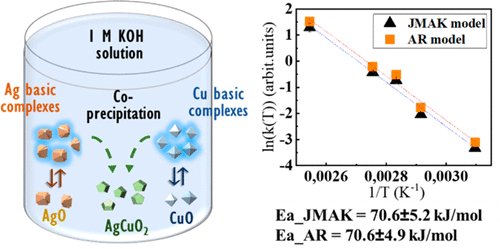
AgCuO2 is an interesting semiconductor oxide with appealing optical and electronic properties. While the oxide has been synthesized in bulk by different approaches, no study of the formation mechanism has been carried out to date. We present an in situ time-resolved X-ray diffraction study of the hydrothermal synthesis of AgCuO2 from AgO and CuO. The effects of reaction pH and temperature on the reaction pathways and products have been studied. While the pH is a key parameter for the successful synthesis of AgCuO2, the temperature affects mainly the reaction kinetics. A reaction pathway is proposed that involves a series of dissolution–precipitation reactions, mediated by Cu and Ag hydroxy complexes. Finally, we have compared different approaches to obtain the reaction activation energy, which was calculated to be 70.6 ± 5.1 kJ/mol. Nevertheless, our results show that new models need to be developed for the type of reaction presented here.
中文翻译:

AgCuO的水热合成反应途径2从原位时间分辨X射线衍射
AgCuO 2是一种有趣的半导体氧化物,具有吸引人的光学和电子特性。尽管已经通过不同方法大量合成了氧化物,但迄今为止尚未对形成机理进行研究。我们提出了从AgO和CuO水热合成AgCuO 2的原位时间分辨X射线衍射研究。研究了反应pH和温度对反应路径和产物的影响。pH是成功合成AgCuO 2的关键参数,温度主要影响反应动力学。提出了一种反应途径,涉及一系列由铜和银羟基配合物介导的溶解-沉淀反应。最后,我们比较了获得反应活化能的不同方法,计算得出的活化能为70.6±5.1 kJ / mol。但是,我们的结果表明,需要针对此处介绍的反应类型开发新的模型。
更新日期:2020-07-01
中文翻译:

AgCuO的水热合成反应途径2从原位时间分辨X射线衍射
AgCuO 2是一种有趣的半导体氧化物,具有吸引人的光学和电子特性。尽管已经通过不同方法大量合成了氧化物,但迄今为止尚未对形成机理进行研究。我们提出了从AgO和CuO水热合成AgCuO 2的原位时间分辨X射线衍射研究。研究了反应pH和温度对反应路径和产物的影响。pH是成功合成AgCuO 2的关键参数,温度主要影响反应动力学。提出了一种反应途径,涉及一系列由铜和银羟基配合物介导的溶解-沉淀反应。最后,我们比较了获得反应活化能的不同方法,计算得出的活化能为70.6±5.1 kJ / mol。但是,我们的结果表明,需要针对此处介绍的反应类型开发新的模型。











































 京公网安备 11010802027423号
京公网安备 11010802027423号