当前位置:
X-MOL 学术
›
Energy Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enhanced Charge Separation and Transfer of Fe2O3@Nitrogen‐Rich Carbon Nitride Tubes for Photocatalytic Water Splitting
Energy Technology ( IF 3.6 ) Pub Date : 2020-04-24 , DOI: 10.1002/ente.202000108 Chen Zhao 1 , Mang Zheng 1 , Dan Wang 1 , Qi Li 1 , Baojiang Jiang 1
Energy Technology ( IF 3.6 ) Pub Date : 2020-04-24 , DOI: 10.1002/ente.202000108 Chen Zhao 1 , Mang Zheng 1 , Dan Wang 1 , Qi Li 1 , Baojiang Jiang 1
Affiliation
|
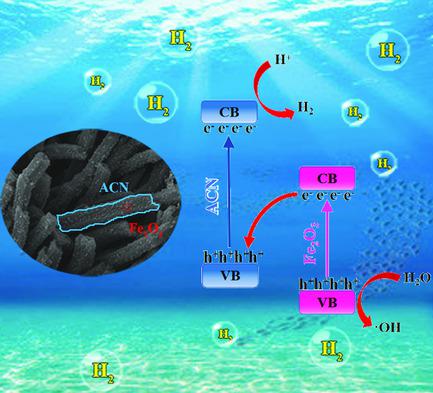
Carbon nitride is widely used in photocatalytic hydrogen production, but it is still difficult to split water without any sacrificial reagent. Herein, nanosized Fe2O3 is combined with 3D nitrogen‐rich carbon nitride tubes (ACN), to form an Fe2O3@ACN Z‐scheme heterojunction, which accelerates the electrons’ transfer from Fe2O3 to ACN and improves the charge separation efficiency. Meanwhile, the bandgap of Fe2O3@ACN is about 2.01 eV, beneficial to the enhancement of visible light absorption capacity. As a result, without any sacrificial agent, the hydrogen evolution rate reaches 3.7 μmol h−1 (10 mg catalyst, AM1.5) through water splitting, which is three times that of ACN and 45 times that of bulk C3N4 (GCN). This work provides a new strategy to prompt pure water splitting based on carbon nitride catalysts.
中文翻译:

Fe2O3 @富氮氮化碳管的电荷分离和转移增强,用于光催化水分解
氮化碳被广泛用于光催化制氢,但是在没有任何牺牲试剂的情况下仍然很难分解水。在本文中,纳米尺寸的Fe 2 ö 3与3D富氮氮化碳管(ACN),结合以形成一种Fe 2 ö 3 @ACN Z-方案异质结,这加速了电子的选自Fe转印2 ö 3至ACN和提高电荷分离效率。同时,Fe 2 O 3 @ACN的带隙约为2.01eV,有利于提高可见光吸收能力。结果,在没有任何牺牲剂的情况下,氢释放速率达到3.7μmolh -1(10 mg催化剂,AM1.5)通过水分解,是ACN的三倍和本体C 3 N 4(GCN)的45倍。这项工作提供了一种基于氮化碳催化剂促进纯水分解的新策略。
更新日期:2020-07-02
中文翻译:

Fe2O3 @富氮氮化碳管的电荷分离和转移增强,用于光催化水分解
氮化碳被广泛用于光催化制氢,但是在没有任何牺牲试剂的情况下仍然很难分解水。在本文中,纳米尺寸的Fe 2 ö 3与3D富氮氮化碳管(ACN),结合以形成一种Fe 2 ö 3 @ACN Z-方案异质结,这加速了电子的选自Fe转印2 ö 3至ACN和提高电荷分离效率。同时,Fe 2 O 3 @ACN的带隙约为2.01eV,有利于提高可见光吸收能力。结果,在没有任何牺牲剂的情况下,氢释放速率达到3.7μmolh -1(10 mg催化剂,AM1.5)通过水分解,是ACN的三倍和本体C 3 N 4(GCN)的45倍。这项工作提供了一种基于氮化碳催化剂促进纯水分解的新策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号