Sustainable Chemistry and Pharmacy ( IF 5.5 ) Pub Date : 2020-04-24 , DOI: 10.1016/j.scp.2020.100258 Adnan Munis , Tianyu Zhao , Maosheng Zheng , Ata Ur Rehman , Feng Wang
|
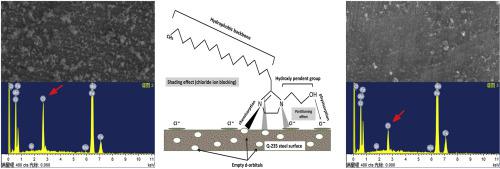
Corrosion and corrosion inhibition behaviors of steel Q-235 were studied in 7.5% NH4Cl solution with the newly synthesized inhibitor by potentiodynamic polarization together with tests of electrochemical impedance spectroscopy and weight loss measurements in 298–338 K temperature range. The new inhibitor 2-(2-heptadecyl-4, 5-dihydro-1H-imidazole-1-yl) ethanol (HDIE) is synthesized with the help of microwave radiations in solvent free conditions. The results showed that inhibition efficiency above 90% is achievable at 298 K with 0.5 mML−1 inhibitor concentration. The Adsorption studies revealed that HDIE follows Langmuir model and its adsorption mechanism was mixed-type with predominant effect on anodic half cell. It was seen from the EDS characterization of corroded steel surfaces that the HDIE retarded the corrosion by impeding the chloride attack. Quantum chemical calculations demonstrated that the imidazoline ring and hetero-atoms were active sites. Moreover, an inhibition mechanism was proposed on the basis of the quantum chemical calculations and experimental corrosion studies.
中文翻译:

在7.5%NH 4 Cl溶液中新合成的绿色缓蚀剂咪唑啉衍生物,用于碳钢
研究了Q-235钢在7.5%NH 4 Cl溶液中和新合成的缓蚀剂的电位动力学极化行为,并在298–338 K温度范围内进行了电化学阻抗谱测试和失重测量。新型抑制剂2-(2-十七烷基-4,5-二氢-1H-咪唑-1-基)乙醇(HDIE)是在无溶剂条件下借助微波辐射合成的。结果表明在0.5 mML -1下在298 K时可达到90%以上的抑制效率抑制剂浓度。吸附研究表明,HDIE遵循Langmuir模型,其吸附机理为混合型,对阳极半电池具有主要作用。从腐蚀的钢表面的EDS表征可以看出,HDIE通过阻止氯化物侵蚀而延迟了腐蚀。量子化学计算表明,咪唑啉环和杂原子是活性位点。此外,在量子化学计算和实验腐蚀研究的基础上,提出了一种缓蚀机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号