当前位置:
X-MOL 学术
›
BBA Mol. Cell Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Overexpression of budding yeast protein phosphatase Ppz1 impairs translation.
Biochimica et Biophysica Acta (BBA) - Molecular Cell Research ( IF 4.6 ) Pub Date : 2020-04-24 , DOI: 10.1016/j.bbamcr.2020.118727 Carlos Calafí 1 , María López-Malo 2 , Diego Velázquez 1 , Chunyi Zhang 2 , José Fernández-Fernández 3 , Olga Rodríguez-Galán 3 , Jesús de la Cruz 3 , Joaquín Ariño 1 , Antonio Casamayor 1
Biochimica et Biophysica Acta (BBA) - Molecular Cell Research ( IF 4.6 ) Pub Date : 2020-04-24 , DOI: 10.1016/j.bbamcr.2020.118727 Carlos Calafí 1 , María López-Malo 2 , Diego Velázquez 1 , Chunyi Zhang 2 , José Fernández-Fernández 3 , Olga Rodríguez-Galán 3 , Jesús de la Cruz 3 , Joaquín Ariño 1 , Antonio Casamayor 1
Affiliation
|
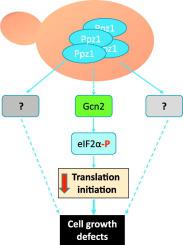
The Ser/Thr protein phosphatase Ppz1 from Saccharomyces cerevisiae is the best characterized member of a family of enzymes only found in fungi. Ppz1 is regulated in vivo by two inhibitory subunits, Hal3 and Vhs3, which are moonlighting proteins also involved in the decarboxylation of the 4-phosphopantothenoylcysteine (PPC) intermediate required for coenzyme A biosynthesis. It has been reported that, when overexpressed, Ppz1 is the most toxic protein in yeast. However, the reasons for such toxicity have not been elucidated. Here we show that the detrimental effect of excessive Ppz1 expression is due to an increase in its phosphatase activity and not to a plausible down-titration of the PPC decarboxylase components. We have identified several genes encoding ribosomal proteins and ribosome assembly factors as mild high-copy suppressors of the toxic Ppz1 effect. Ppz1 binds to ribosomes engaged in translation and copurifies with diverse ribosomal proteins and translation factors. Ppz1 overexpression results in Gcn2-dependent increased phosphorylation of eIF2α at Ser-51. Consistently, deletion of GCN2 partially suppresses the growth defect of a Ppz1 overexpressing strain. We propose that the deleterious effects of Ppz1 overexpression are in part due to alteration in normal protein synthesis.
中文翻译:

出芽的酵母蛋白磷酸酶Ppz1的过表达损害翻译。
来自酿酒酵母的Ser / Thr蛋白磷酸酶Ppz1是仅在真菌中发现的酶家族中最有特色的成员。Ppz1在体内受两个抑制亚基Hal3和Vhs3的调节,它们是月光下的蛋白质,也参与辅酶A生物合成所需的4-磷酸戊二烯酰半胱氨酸(PPC)中间体的脱羧作用。据报道,当过表达时,Ppz1是酵母中毒性最高的蛋白质。但是,尚未阐明产生这种毒性的原因。在这里,我们表明过量Ppz1表达的有害作用是由于其磷酸酶活性的增加,而不是由于PPC脱羧酶组分的可能的滴定。我们已经确定了几个编码核糖体蛋白和核糖体装配因子的基因作为毒性Ppz1效应的轻度高拷贝抑制剂。Ppz1与参与翻译的核糖体结合,并与多种核糖体蛋白和翻译因子共纯化。Ppz1过表达导致Ser-51上eIF2α的Gcn2依赖性磷酸化增加。一致地,GCN2的删除部分地抑制了Ppz1过表达菌株的生长缺陷。我们建议Ppz1过表达的有害影响部分是由于正常蛋白质合成的改变。GCN2的缺失部分抑制了Ppz1过表达菌株的生长缺陷。我们建议Ppz1过表达的有害影响部分是由于正常蛋白质合成的改变。GCN2的缺失部分抑制了Ppz1过表达菌株的生长缺陷。我们建议Ppz1过表达的有害影响部分是由于正常蛋白质合成的改变。
更新日期:2020-04-24
中文翻译:

出芽的酵母蛋白磷酸酶Ppz1的过表达损害翻译。
来自酿酒酵母的Ser / Thr蛋白磷酸酶Ppz1是仅在真菌中发现的酶家族中最有特色的成员。Ppz1在体内受两个抑制亚基Hal3和Vhs3的调节,它们是月光下的蛋白质,也参与辅酶A生物合成所需的4-磷酸戊二烯酰半胱氨酸(PPC)中间体的脱羧作用。据报道,当过表达时,Ppz1是酵母中毒性最高的蛋白质。但是,尚未阐明产生这种毒性的原因。在这里,我们表明过量Ppz1表达的有害作用是由于其磷酸酶活性的增加,而不是由于PPC脱羧酶组分的可能的滴定。我们已经确定了几个编码核糖体蛋白和核糖体装配因子的基因作为毒性Ppz1效应的轻度高拷贝抑制剂。Ppz1与参与翻译的核糖体结合,并与多种核糖体蛋白和翻译因子共纯化。Ppz1过表达导致Ser-51上eIF2α的Gcn2依赖性磷酸化增加。一致地,GCN2的删除部分地抑制了Ppz1过表达菌株的生长缺陷。我们建议Ppz1过表达的有害影响部分是由于正常蛋白质合成的改变。GCN2的缺失部分抑制了Ppz1过表达菌株的生长缺陷。我们建议Ppz1过表达的有害影响部分是由于正常蛋白质合成的改变。GCN2的缺失部分抑制了Ppz1过表达菌株的生长缺陷。我们建议Ppz1过表达的有害影响部分是由于正常蛋白质合成的改变。











































 京公网安备 11010802027423号
京公网安备 11010802027423号