当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Proteomic analysis of the S. cerevisiae response to the anticancer ruthenium complex KP1019.
Metallomics ( IF 2.9 ) Pub Date : 2020-04-24 , DOI: 10.1039/d0mt00008f Laura K Stultz 1 , Alexandra Hunsucker 2 , Sydney Middleton 1 , Evan Grovenstein 2 , Jacob O'Leary 1 , Eliot Blatt 3 , Mary Miller 3 , James Mobley 4 , Pamela K Hanson 5
Metallomics ( IF 2.9 ) Pub Date : 2020-04-24 , DOI: 10.1039/d0mt00008f Laura K Stultz 1 , Alexandra Hunsucker 2 , Sydney Middleton 1 , Evan Grovenstein 2 , Jacob O'Leary 1 , Eliot Blatt 3 , Mary Miller 3 , James Mobley 4 , Pamela K Hanson 5
Affiliation
|
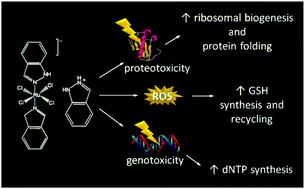
Like platinum-based chemotherapeutics, the anticancer ruthenium complex indazolium trans-[tetrachlorobis(1H-indazole)ruthenate(III)], or KP1019, damages DNA, induces apoptosis, and causes tumor regression in animal models. Unlike platinum-based drugs, KP1019 showed no dose-limiting toxicity in a phase I clinical trial. Despite these advances, the mechanism(s) and target(s) of KP1019 remain unclear. For example, the drug may damage DNA directly or by causing oxidative stress. Likewise, KP1019 binds cytosolic proteins, suggesting DNA is not the sole target. Here we use the budding yeast Saccharomyces cerevisiae as a model in a proteomic study of the cellular response to KP1019. Mapping protein level changes onto metabolic pathways revealed patterns consistent with elevated synthesis and/or cycling of the antioxidant glutathione, suggesting KP1019 induces oxidative stress. This result was supported by increased fluorescence of the redox-sensitive dye DCFH-DA and increased KP1019 sensitivity of yeast lacking Yap1, a master regulator of the oxidative stress response. In addition to oxidative and DNA stress, bioinformatic analysis revealed drug-dependent increases in proteins involved ribosome biogenesis, translation, and protein (re)folding. Consistent with proteotoxic effects, KP1019 increased expression of a heat-shock element (HSE) lacZ reporter. KP1019 pre-treatment also sensitized yeast to oxaliplatin, paralleling prior research showing that cancer cell lines with elevated levels of translation machinery are hypersensitive to oxaliplatin. Combined, these data suggest that one of KP1019's many targets may be protein metabolism, which opens up intriguing possibilities for combination therapy.
中文翻译:

酿酒酵母对抗癌钌复合物 KP1019 反应的蛋白质组学分析。
与铂类化疗药物一样,抗癌钌络合物吲唑钌反式-[四氯双( 1H-吲唑)钌( III )]或KP1019在动物模型中损伤DNA、诱导细胞凋亡并导致肿瘤消退。与铂类药物不同,KP1019 在 I 期临床试验中没有显示出剂量限制性毒性。尽管取得了这些进展,KP1019 的机制和目标仍不清楚。例如,药物可能会直接损伤 DNA 或通过引起氧化应激。同样,KP1019 结合胞浆蛋白,表明 DNA 不是唯一的目标。在这里,我们使用芽殖酵母酿酒酵母作为 KP1019 细胞反应的蛋白质组学研究模型。将蛋白质水平变化映射到代谢途径揭示了与抗氧化剂谷胱甘肽合成和/或循环升高一致的模式,表明 KP1019 会诱导氧化应激。氧化还原敏感染料 DCFH-DA 的荧光增加以及缺乏 Yap1(氧化应激反应的主要调节因子)的酵母 KP1019 敏感性增加支持了这一结果。除了氧化和 DNA 应激之外,生物信息学分析还揭示了药物依赖性蛋白质增加涉及核糖体生物发生、翻译和蛋白质(重新)折叠。与蛋白毒性作用一致,KP1019 增加了热休克元件 (HSE) lacZ报告基因的表达。 KP1019 预处理还使酵母对奥沙利铂敏感,与先前的研究相一致,该研究表明翻译机制水平升高的癌细胞系对奥沙利铂高度敏感。综合起来,这些数据表明 KP1019 的众多靶点之一可能是蛋白质代谢,这为联合治疗开辟了有趣的可能性。
更新日期:2020-06-25
中文翻译:

酿酒酵母对抗癌钌复合物 KP1019 反应的蛋白质组学分析。
与铂类化疗药物一样,抗癌钌络合物吲唑钌反式-[四氯双( 1H-吲唑)钌( III )]或KP1019在动物模型中损伤DNA、诱导细胞凋亡并导致肿瘤消退。与铂类药物不同,KP1019 在 I 期临床试验中没有显示出剂量限制性毒性。尽管取得了这些进展,KP1019 的机制和目标仍不清楚。例如,药物可能会直接损伤 DNA 或通过引起氧化应激。同样,KP1019 结合胞浆蛋白,表明 DNA 不是唯一的目标。在这里,我们使用芽殖酵母酿酒酵母作为 KP1019 细胞反应的蛋白质组学研究模型。将蛋白质水平变化映射到代谢途径揭示了与抗氧化剂谷胱甘肽合成和/或循环升高一致的模式,表明 KP1019 会诱导氧化应激。氧化还原敏感染料 DCFH-DA 的荧光增加以及缺乏 Yap1(氧化应激反应的主要调节因子)的酵母 KP1019 敏感性增加支持了这一结果。除了氧化和 DNA 应激之外,生物信息学分析还揭示了药物依赖性蛋白质增加涉及核糖体生物发生、翻译和蛋白质(重新)折叠。与蛋白毒性作用一致,KP1019 增加了热休克元件 (HSE) lacZ报告基因的表达。 KP1019 预处理还使酵母对奥沙利铂敏感,与先前的研究相一致,该研究表明翻译机制水平升高的癌细胞系对奥沙利铂高度敏感。综合起来,这些数据表明 KP1019 的众多靶点之一可能是蛋白质代谢,这为联合治疗开辟了有趣的可能性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号