当前位置:
X-MOL 学术
›
Int. J. Quantum Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical examination of covalency in berkelium(IV) carbonate complexes
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2020-04-23 , DOI: 10.1002/qua.26254 Thomas E. Albrecht‐Schmitt 1 , David E. Hobart 1 , Dayan Páez‐Hernández 2 , Cristian Celis‐Barros 1
International Journal of Quantum Chemistry ( IF 2.3 ) Pub Date : 2020-04-23 , DOI: 10.1002/qua.26254 Thomas E. Albrecht‐Schmitt 1 , David E. Hobart 1 , Dayan Páez‐Hernández 2 , Cristian Celis‐Barros 1
Affiliation
|
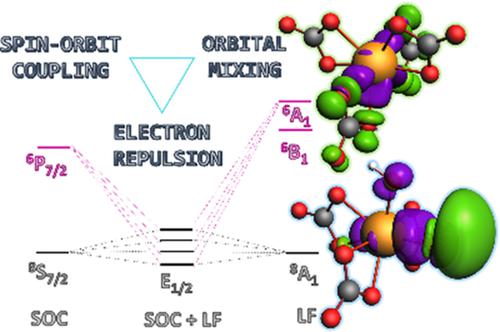
Experimental studies on the speciation of berkelium in carbonate media have shown that complexation of berkelium(III) by carbonate results in spontaneous oxidation to berkelium(IV) and that multiple species can be present in solution. We studied two proposed structures present in solution based on theoretical comparisons with spectroscopic data previously reported for Bk(IV) carbonate solutions. The multiconfigurational character of the ground and low‐lying excited states in both complexes is demonstrated to result from the strong spin‐orbit coupling. Although bonding in Bk(IV) carbonate and carbonate‐hydroxide complexes is dominated by strong Coulombic forces, the presence of non‐negligible covalent character is supported by ligand‐field theory, natural localized orbitals, topological studies of the electron density, and energy transition state natural orbitals for chemical valence. Bond orders based on natural localized molecular orbitals show that BkOH bonds possess enhanced orbital overlap, which is reflected in the bond strength. This is also observed in the decomposition of the orbital interaction energy into individual deformation density pairs.
中文翻译:

碳酸铍(IV)配合物的理论价研究
对碳酸盐介质中铍形态的实验研究表明,碳酸盐将铍(III)络合会导致自发氧化为铍(IV),并且溶液中可能存在多种物质。我们基于理论比较与先前报道的Bk(IV)碳酸盐溶液的光谱数据,研究了溶液中存在的两种拟议结构。两种复合物中基态和低激发态的多构型特征都证明是强自旋轨道耦合造成的。尽管在Bk(IV)碳酸盐和碳酸盐-氢氧化物络合物中的键合以强大的库仑力为主,但配体场论,自然局部轨道,电子密度的拓扑研究支持了不可忽略的共价特征。和能量跃迁状态的化学价自然轨道。基于自然局部分子轨道的键序表明BkOH键具有增强的轨道重叠,这在键强度中得到反映。在将轨道相互作用能分解成单独的变形密度对时,也可以观察到这一点。
更新日期:2020-04-23
中文翻译:

碳酸铍(IV)配合物的理论价研究
对碳酸盐介质中铍形态的实验研究表明,碳酸盐将铍(III)络合会导致自发氧化为铍(IV),并且溶液中可能存在多种物质。我们基于理论比较与先前报道的Bk(IV)碳酸盐溶液的光谱数据,研究了溶液中存在的两种拟议结构。两种复合物中基态和低激发态的多构型特征都证明是强自旋轨道耦合造成的。尽管在Bk(IV)碳酸盐和碳酸盐-氢氧化物络合物中的键合以强大的库仑力为主,但配体场论,自然局部轨道,电子密度的拓扑研究支持了不可忽略的共价特征。和能量跃迁状态的化学价自然轨道。基于自然局部分子轨道的键序表明BkOH键具有增强的轨道重叠,这在键强度中得到反映。在将轨道相互作用能分解成单独的变形密度对时,也可以观察到这一点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号