当前位置:
X-MOL 学术
›
BBA Gen. Subj.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NDRG1 suppresses basal and hypoxia-induced autophagy at both the initiation and degradation stages and sensitizes pancreatic cancer cells to lysosomal membrane permeabilization.
Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 2.8 ) Pub Date : 2020-04-23 , DOI: 10.1016/j.bbagen.2020.129625 Sumit Sahni 1 , Josef Gillson 1 , Kyung Chan Park 2 , Shannon Chiang 2 , Lionel Yi Wen Leck 3 , Patric J Jansson 3 , Des R Richardson 4
Biochimica et Biophysica Acta (BBA) - General Subjects ( IF 2.8 ) Pub Date : 2020-04-23 , DOI: 10.1016/j.bbagen.2020.129625 Sumit Sahni 1 , Josef Gillson 1 , Kyung Chan Park 2 , Shannon Chiang 2 , Lionel Yi Wen Leck 3 , Patric J Jansson 3 , Des R Richardson 4
Affiliation
|
BACKGROUND
N-myc downstream regulated gene 1 (NDRG1) is an established stress-response protein. This study investigated the effects of NDRG1 on autophagic degradation and how this can be therapeutically exploited.
METHODS
Cell culture, western analysis, confocal microscopy, acridine orange staining, cholesterol determination, cellular proliferation assessment and combination index (CI) estimation.
RESULTS
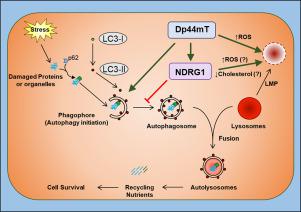
NDRG1 expression suppressed autophagic degradation and autolysosome formation, measured by increased p62 expression and reduced co-localization between the well-characterized, autophagosomal and lysosomal markers, LC3 and LAMP2, respectively. NDRG1 elicited autophagic suppression at the initiation stage of autophagy. The NDRG1-inducer and anti-cancer agent, di-2-pyridylketone 4,4,-dimethyl-3-thiosemicarbazone (Dp44mT), was able to induce lysosomal membrane permeabilization (LMP). Over-expression of NDRG1 further sensitized cells to LMP mediated by both Dp44mT, or the redox active Dp44mT‑copper complex. This sensitization may be mediated via a decrease in cholesterol levels upon NDRG1 expression, as cholesterol stabilizes lysosomal membranes. However, the effect of NDRG1 on cholesterol appeared independent of the key energy homeostasis sensor, 5' AMP-activated protein kinase (AMPK), whose activation was significantly (p < 0.001) reduced by NDRG1. Finally, Dp44mT synergistically potentiated the anti-proliferative activity of Gemcitabine that activates autophagy. In fact, Dp44mT and Gemcitabine (Combination Index (CI): 0.38 ± 0.07) demonstrated higher synergism versus the autophagy inhibitor, Bafilomycin A1 and Gemcitabine (CI: 0.64 ± 0.19).
CONCLUSIONS AND GENERAL SIGNIFICANCE
Collectively, this study demonstrated a dual-inhibitory mechanism of NDRG1 on autophagic activity, and that NDRG1 expression sensitized cells to Dp44mT-induced LMP. Considering the ability of Dp44mT to inhibit autophagy, studies demonstrated the potential of combination therapy for cancer treatment of Dp44mT with Gemcitabine.
中文翻译:

NDRG1在启动和降解阶段均抑制基础和缺氧诱导的自噬,并使胰腺癌细胞对溶酶体膜通透性敏感。
背景技术N-myc下游调控基因1(NDRG1)是已建立的应激反应蛋白。这项研究调查了NDRG1对自噬降解的影响以及如何对其进行治疗性利用。方法细胞培养,Western分析,共聚焦显微镜,a啶橙染色,胆固醇测定,细胞增殖评估和组合指数(CI)估计。结果NDRG1的表达抑制了自噬降解和自溶酶体的形成,这是通过增加p62的表达和减少分别表征的,自噬体和溶酶体标记物LC3和LAMP2之间的共定位来衡量的。NDRG1在自噬的起始阶段引起自噬抑制。NDRG1诱导剂和抗癌药,二-2-吡啶基酮4,4,-二甲基-3-硫代半脲(Dp44mT),能够诱导溶酶体膜通透性(LMP)。NDRG1的过表达进一步使细胞对Dp44mT或氧化还原活性Dp44mT-铜复合物介导的LMP敏感。这种敏感性可能是由于NDRG1表达降低胆固醇水平而介导的,因为胆固醇稳定了溶酶体膜。然而,NDRG1对胆固醇的作用似乎与关键能量稳态传感器5'AMP激活的蛋白激酶(AMPK)无关,NDRG1的激活显着降低(p <0.001)。最后,Dp44mT协同增强了吉西他滨激活自噬的抗增殖活性。实际上,Dp44mT和吉西他滨(组合指数(CI):0.38±0.07)显示出比自噬抑制剂巴氟霉素A1和吉西他滨更高的协同作用(CI:0.64±0.19)。结论和一般意义上,这项研究证明了NDRG1对自噬活性的双重抑制机制,并且NDRG1的表达使细胞对Dp44mT诱导的LMP敏感。考虑到Dp44mT抑制自噬的能力,研究证明吉西他滨联合治疗Dp44mT的癌症治疗潜力。
更新日期:2020-04-23
中文翻译:

NDRG1在启动和降解阶段均抑制基础和缺氧诱导的自噬,并使胰腺癌细胞对溶酶体膜通透性敏感。
背景技术N-myc下游调控基因1(NDRG1)是已建立的应激反应蛋白。这项研究调查了NDRG1对自噬降解的影响以及如何对其进行治疗性利用。方法细胞培养,Western分析,共聚焦显微镜,a啶橙染色,胆固醇测定,细胞增殖评估和组合指数(CI)估计。结果NDRG1的表达抑制了自噬降解和自溶酶体的形成,这是通过增加p62的表达和减少分别表征的,自噬体和溶酶体标记物LC3和LAMP2之间的共定位来衡量的。NDRG1在自噬的起始阶段引起自噬抑制。NDRG1诱导剂和抗癌药,二-2-吡啶基酮4,4,-二甲基-3-硫代半脲(Dp44mT),能够诱导溶酶体膜通透性(LMP)。NDRG1的过表达进一步使细胞对Dp44mT或氧化还原活性Dp44mT-铜复合物介导的LMP敏感。这种敏感性可能是由于NDRG1表达降低胆固醇水平而介导的,因为胆固醇稳定了溶酶体膜。然而,NDRG1对胆固醇的作用似乎与关键能量稳态传感器5'AMP激活的蛋白激酶(AMPK)无关,NDRG1的激活显着降低(p <0.001)。最后,Dp44mT协同增强了吉西他滨激活自噬的抗增殖活性。实际上,Dp44mT和吉西他滨(组合指数(CI):0.38±0.07)显示出比自噬抑制剂巴氟霉素A1和吉西他滨更高的协同作用(CI:0.64±0.19)。结论和一般意义上,这项研究证明了NDRG1对自噬活性的双重抑制机制,并且NDRG1的表达使细胞对Dp44mT诱导的LMP敏感。考虑到Dp44mT抑制自噬的能力,研究证明吉西他滨联合治疗Dp44mT的癌症治疗潜力。









































 京公网安备 11010802027423号
京公网安备 11010802027423号