当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient Sortase-Mediated Ligation Using a Common C-Terminal Fusion Tag.
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-04-23 , DOI: 10.1021/acs.bioconjchem.0c00156 Sierra A Reed 1 , David A Brzovic 1 , Savanna S Takasaki 1 , Kristina V Boyko 1 , John M Antos 1
Bioconjugate Chemistry ( IF 4.0 ) Pub Date : 2020-04-23 , DOI: 10.1021/acs.bioconjchem.0c00156 Sierra A Reed 1 , David A Brzovic 1 , Savanna S Takasaki 1 , Kristina V Boyko 1 , John M Antos 1
Affiliation
|
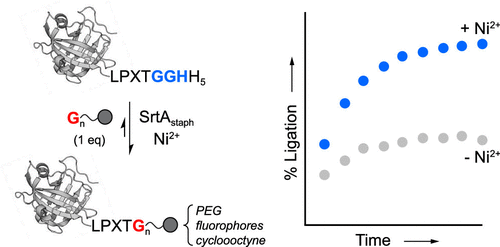
Sortase-mediated ligation is a powerful method for generating site-specifically modified proteins. However, this process is limited by the inherent reversibility of the ligation reaction. To address this, here we report the continued development and optimization of an experimentally facile strategy for blocking reaction reversibility. This approach, which we have termed metal-assisted sortase-mediated ligation (MA-SML), relies on the use of a solution additive (Ni2+) and a C-terminal tag (LPXTGGHH5) that is widely used for converting protein targets into sortase substrates. In a series of model systems utilizing a 1:1 molar ratio of sortase substrate and glycine amine nucleophile, we find that MA-SML consistently improves the extent of ligation. This enables the modification of proteins with fluorophores, PEG, and a bioorthogonal cyclooctyne moiety without the need to use precious reagents in excess. Overall, these results demonstrate the potential of MA-SML as a general strategy for improving reaction efficiency in a broad range of sortase-based protein engineering applications.
中文翻译:

使用通用 C 端融合标签进行高效分选酶介导的连接。
分选酶介导的连接是产生位点特异性修饰蛋白质的有效方法。然而,该过程受到连接反应固有的可逆性的限制。为了解决这个问题,我们在这里报告了一种实验简便的阻断反应可逆性策略的持续开发和优化。我们将这种方法称为金属辅助分选酶介导的连接 (MA-SML),它依赖于溶液添加剂 (Ni2+) 和 C 末端标签 (LPXTGGHH5) 的使用,广泛用于将蛋白质靶标转化为分选酶基材。在一系列使用摩尔比为 1:1 的分选酶底物和甘氨酸胺亲核试剂的模型系统中,我们发现 MA-SML 持续改善了连接程度。这使得能够用荧光团、PEG 和生物正交环辛炔部分修饰蛋白质,而无需使用过量的珍贵试剂。总体而言,这些结果证明了 MA-SML 作为提高各种基于分选酶的蛋白质工程应用中反应效率的通用策略的潜力。
更新日期:2020-04-23
中文翻译:

使用通用 C 端融合标签进行高效分选酶介导的连接。
分选酶介导的连接是产生位点特异性修饰蛋白质的有效方法。然而,该过程受到连接反应固有的可逆性的限制。为了解决这个问题,我们在这里报告了一种实验简便的阻断反应可逆性策略的持续开发和优化。我们将这种方法称为金属辅助分选酶介导的连接 (MA-SML),它依赖于溶液添加剂 (Ni2+) 和 C 末端标签 (LPXTGGHH5) 的使用,广泛用于将蛋白质靶标转化为分选酶基材。在一系列使用摩尔比为 1:1 的分选酶底物和甘氨酸胺亲核试剂的模型系统中,我们发现 MA-SML 持续改善了连接程度。这使得能够用荧光团、PEG 和生物正交环辛炔部分修饰蛋白质,而无需使用过量的珍贵试剂。总体而言,这些结果证明了 MA-SML 作为提高各种基于分选酶的蛋白质工程应用中反应效率的通用策略的潜力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号