Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Peptidylprolylisomerases, Protein Folders, or Scaffolders? The Example of FKBP51 and FKBP52.
BioEssays ( IF 3.2 ) Pub Date : 2020-04-22 , DOI: 10.1002/bies.201900250 Theo Rein 1
BioEssays ( IF 3.2 ) Pub Date : 2020-04-22 , DOI: 10.1002/bies.201900250 Theo Rein 1
Affiliation
|
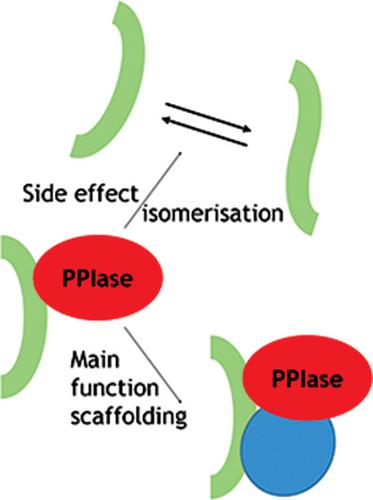
Peptidylprolyl‐isomerases (PPIases) comprise of the protein families of FK506 binding proteins (FKBPs), cyclophilins, and parvulins. Their common feature is their ability to expedite the transition of peptidylprolyl bonds between the cis and the trans conformation. Thus, it seemed highly plausible that PPIase enzymatic activity is crucial for protein folding. However, this has been difficult to prove over the decades since their discovery. In parallel, more and more studies have discovered scaffolding functions of PPIases. This essay discusses the hypothesis that PPIase enzymatic activity might be the consequence of binding to peptidylprolyl protein motifs. The main focus of this paper is the large immunophilins FKBP51 and FKBP52, but other PPIases such as cyclophilin A and Pin1 are also described. From the hypothesis, it follows that the PPIase activity of these proteins might be less relevant, if at all, than the organization of protein complexes through versatile protein binding. Also see the video abstract here https://youtu.be/A33la0dx5LE.
中文翻译:

肽基脯氨酰异构酶、蛋白质文件夹或支架?FKBP51 和 FKBP52 的示例。
肽基脯氨酰异构酶 (PPIases) 由 FK506 结合蛋白 (FKBPs)、亲环蛋白和细小蛋白的蛋白质家族组成。它们的共同特点是能够加快顺式和反式之间肽基脯氨酰键的转变。构象。因此,PPIase 酶活性对于蛋白质折叠至关重要。然而,自发现以来的几十年里,这一直难以证明。与此同时,越来越多的研究发现了 PPIases 的支架功能。本文讨论了 PPIase 酶活性可能是与肽基脯氨酰蛋白基序结合的结果的假设。本文的主要焦点是大的亲免蛋白 FKBP51 和 FKBP52,但也描述了其他 PPIase,如亲环蛋白 A 和 Pin1。根据假设,这些蛋白质的 PPIase 活性可能比通过多功能蛋白质结合组织蛋白质复合物的相关性更小(如果有的话)。另请参阅此处的视频摘要 https://youtu.be/A33la0dx5LE。
更新日期:2020-06-29
中文翻译:

肽基脯氨酰异构酶、蛋白质文件夹或支架?FKBP51 和 FKBP52 的示例。
肽基脯氨酰异构酶 (PPIases) 由 FK506 结合蛋白 (FKBPs)、亲环蛋白和细小蛋白的蛋白质家族组成。它们的共同特点是能够加快顺式和反式之间肽基脯氨酰键的转变。构象。因此,PPIase 酶活性对于蛋白质折叠至关重要。然而,自发现以来的几十年里,这一直难以证明。与此同时,越来越多的研究发现了 PPIases 的支架功能。本文讨论了 PPIase 酶活性可能是与肽基脯氨酰蛋白基序结合的结果的假设。本文的主要焦点是大的亲免蛋白 FKBP51 和 FKBP52,但也描述了其他 PPIase,如亲环蛋白 A 和 Pin1。根据假设,这些蛋白质的 PPIase 活性可能比通过多功能蛋白质结合组织蛋白质复合物的相关性更小(如果有的话)。另请参阅此处的视频摘要 https://youtu.be/A33la0dx5LE。











































 京公网安备 11010802027423号
京公网安备 11010802027423号