Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformational selection in the flaviviral NS2B-NS3 protease.
Biochimie ( IF 3.3 ) Pub Date : 2020-04-23 , DOI: 10.1016/j.biochi.2020.04.014 Mira A M Behnam 1 , Christian D P Klein 1
Biochimie ( IF 3.3 ) Pub Date : 2020-04-23 , DOI: 10.1016/j.biochi.2020.04.014 Mira A M Behnam 1 , Christian D P Klein 1
Affiliation
|
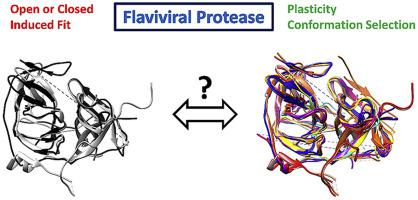
The first x-ray structures of flaviviral proteases defined two conformational states, open and closed, depending on the relative position of NS2B with respect to NS3, a feature that affects the shape of the binding site. The degree of flexibility in the active site was limited to changes in the fold of NS2B rather than NS3 and an induced-fit mechanism was regarded as the main factor for ligand binding. A minor degree of conformational plasticity in NS3 is observed in the two protein chains in the asymmetric unit for the structure of Zika protease with a dipeptide boronate, synthesized in our group. We hypothesize that the NS3 fold has a crucial influence on the shape of the binding site and that a reevaluation of the induced-fit interpretation is warranted. A comparison of flaviviral protease structures identifies conformational dynamics of NS3 and their unexpected role in controlling the depth of the, otherwise shallow, active site. The structural changes of NS3 are mediated by conserved residues and reveal a subpocket, which we denote as subpocket B, extending beyond the catalytic aspartate 75 towards the allosteric binding site, providing a unique connection between the orthosteric and allosteric sites in the protease. The structural evidence supports a molecular recognition based primarily on conformational selection and population shift rather than induced-fit. Besides the implications on protease studies and drug development, this hypothesis provides an interpretation for the alternate binding modes with respect to the catalytic serine, which are observed for recently developed beta-lactam inhibitors incorporating benzyloxyphenylglycine.
中文翻译:

黄病毒NS2B-NS3蛋白酶的构象选择。
根据NS2B相对于NS3的相对位置(影响结合位点形状的特征),黄病毒病毒蛋白酶的第一个X射线结构定义了两个构象状态,即打开和关闭。活性位点的柔性程度限于NS2B而不是NS3的倍数变化,并且诱导拟合机制被认为是配体结合的主要因素。在我们小组中合成的Zika蛋白酶与二肽硼酸酯的不对称单元的两条蛋白质链中,观察到NS3的构象可塑性较小。我们假设NS3折叠对结合位点的形状具有至关重要的影响,并且有必要对诱导拟合解释进行重新评估。黄病毒蛋白酶结构的比较可确定NS3的构象动力学及其在控制活性位点(否则为浅点)的深度中的意外作用。NS3的结构变化是由保守的残基介导的,并揭示了一个亚口袋,我们称为亚口袋B,它延伸超出催化天冬氨酸75并朝向变构结合位点,从而在蛋白酶的正构和变构位点之间提供了独特的联系。结构证据支持分子识别主要基于构象选择和种群转移而不是诱导拟合。除了对蛋白酶研究和药物开发有影响外,该假设还为催化丝氨酸的其他结合方式提供了解释,
更新日期:2020-04-23
中文翻译:

黄病毒NS2B-NS3蛋白酶的构象选择。
根据NS2B相对于NS3的相对位置(影响结合位点形状的特征),黄病毒病毒蛋白酶的第一个X射线结构定义了两个构象状态,即打开和关闭。活性位点的柔性程度限于NS2B而不是NS3的倍数变化,并且诱导拟合机制被认为是配体结合的主要因素。在我们小组中合成的Zika蛋白酶与二肽硼酸酯的不对称单元的两条蛋白质链中,观察到NS3的构象可塑性较小。我们假设NS3折叠对结合位点的形状具有至关重要的影响,并且有必要对诱导拟合解释进行重新评估。黄病毒蛋白酶结构的比较可确定NS3的构象动力学及其在控制活性位点(否则为浅点)的深度中的意外作用。NS3的结构变化是由保守的残基介导的,并揭示了一个亚口袋,我们称为亚口袋B,它延伸超出催化天冬氨酸75并朝向变构结合位点,从而在蛋白酶的正构和变构位点之间提供了独特的联系。结构证据支持分子识别主要基于构象选择和种群转移而不是诱导拟合。除了对蛋白酶研究和药物开发有影响外,该假设还为催化丝氨酸的其他结合方式提供了解释,











































 京公网安备 11010802027423号
京公网安备 11010802027423号