当前位置:
X-MOL 学术
›
Cell Chem. Bio.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Combined Omics Approach Identifies Gambogic Acid and Related Xanthones as Covalent Inhibitors of the Serine Palmitoyltransferase Complex.
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2020-04-23 , DOI: 10.1016/j.chembiol.2020.03.008 Dominic G Hoch 1 , Daniel Abegg 2 , J Thomas Hannich 3 , Dany Pechalrieu 2 , Anton Shuster 2 , Brendan G Dwyer 2 , Chao Wang 4 , Xiaojin Zhang 5 , Qidong You 6 , Howard Riezman 3 , Alexander Adibekian 2
Cell Chemical Biology ( IF 6.6 ) Pub Date : 2020-04-23 , DOI: 10.1016/j.chembiol.2020.03.008 Dominic G Hoch 1 , Daniel Abegg 2 , J Thomas Hannich 3 , Dany Pechalrieu 2 , Anton Shuster 2 , Brendan G Dwyer 2 , Chao Wang 4 , Xiaojin Zhang 5 , Qidong You 6 , Howard Riezman 3 , Alexander Adibekian 2
Affiliation
|
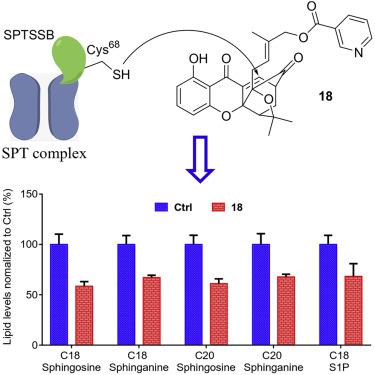
In this study, we identify the natural product gambogic acid as well as structurally related synthetic xanthones as first-in-class covalent inhibitors of the de novo sphingolipid biosynthesis. We apply chemoproteomics to determine that gambogic acid binds to the regulatory small subunit B of the serine palmitoyltransferase complex (SPTSSB). We then test structurally related synthetic xanthones to identify 18 as an equally potent but more selective binder of SPTSSB and show that 18 reduces sphingolipid levels in situ and in vivo. Finally, using various biological methods, we demonstrate that 18 induces cellular responses characteristic for diminished sphingosine-1-phosphate (S1P) signaling. This study demonstrates that SPTSSB may become a viable therapeutic target in various diseases with pathological S1P signaling. Furthermore, we believe that our compound will become a valuable tool for studying the sphingolipid metabolism and serve as a blueprint for the development of a new generation of sphingolipid biosynthesis inhibitors.
中文翻译:

组合的组学方法将藤黄酸和相关的氧杂蒽酮确定为丝氨酸棕榈酰转移酶复合物的共价抑制剂。
在这项研究中,我们将天然产物藤黄酸以及与结构相关的合成氧杂蒽酮确定为从头鞘脂生物合成的同类共价抑制剂。我们应用化学蛋白质组学来确定藤黄酸与丝氨酸棕榈酰转移酶复合物(SPTSSB)的调节小亚基B结合。然后,我们测试与结构相关的合成氧杂蒽酮,以鉴定18个与SPTSSB具有同等效力但更具选择性的结合剂,并显示18降低了原位和体内鞘脂的水平。最后,使用各种生物学方法,我们证明18诱导特征性鞘氨醇-1-磷酸(S1P)信号转导减少的细胞反应。这项研究表明,SPTSSB可能成为具有病理性S1P信号传导的各种疾病的可行治疗靶标。此外,
更新日期:2020-04-23
中文翻译:

组合的组学方法将藤黄酸和相关的氧杂蒽酮确定为丝氨酸棕榈酰转移酶复合物的共价抑制剂。
在这项研究中,我们将天然产物藤黄酸以及与结构相关的合成氧杂蒽酮确定为从头鞘脂生物合成的同类共价抑制剂。我们应用化学蛋白质组学来确定藤黄酸与丝氨酸棕榈酰转移酶复合物(SPTSSB)的调节小亚基B结合。然后,我们测试与结构相关的合成氧杂蒽酮,以鉴定18个与SPTSSB具有同等效力但更具选择性的结合剂,并显示18降低了原位和体内鞘脂的水平。最后,使用各种生物学方法,我们证明18诱导特征性鞘氨醇-1-磷酸(S1P)信号转导减少的细胞反应。这项研究表明,SPTSSB可能成为具有病理性S1P信号传导的各种疾病的可行治疗靶标。此外,











































 京公网安备 11010802027423号
京公网安备 11010802027423号