Communications Chemistry ( IF 5.9 ) Pub Date : 2020-04-21 , DOI: 10.1038/s42004-020-0294-1 Ester Livshits 1 , Itamar Luzon 1 , Krishnendu Gope 1 , Roi Baer 2 , Daniel Strasser 1
|
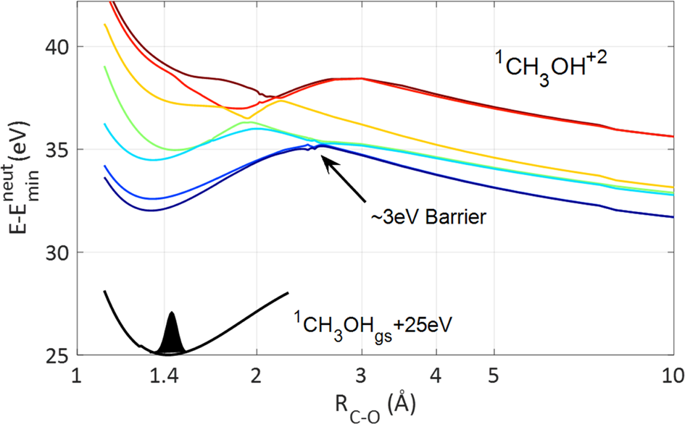
The time scales and formation mechanisms of tri-hydrogen cation products in organic molecule ionization processes are poorly understood, despite their cardinal role in the chemistry of the interstellar medium and in other chemical systems. Using an ultrafast extreme-ultraviolet pump and time-resolved near-IR probe, combined with high-level ab initio molecular dynamics calculations, here we report unambiguously that H3+ formation in double-ionization of methanol occurs on a sub 100 fs time scale, settling previous conflicting findings of strong-field Coulomb explosion experiments. Our combined experimental–computational studies suggest that ultrafast competition, between proton-transfer and long-range electron-transfer processes, determines whether the roaming neutral H2 dynamics on the dication result in \({\mathrm{H}}_3^ +\) or \({\mathrm{H}}_2^ +\) fragments respectively.
中文翻译:

使用极紫外脉冲对超快 H 2 漫游化学和 H 3 + 形成进行时间分辨
三氢阳离子产物在有机分子电离过程中的时间尺度和形成机制知之甚少,尽管它们在星际介质和其他化学系统的化学中起着重要作用。使用超快极紫外泵和时间分辨近红外探针,结合高级从头算分子动力学计算,我们在这里明确报告甲醇双电离中 H 3 +的形成发生在 100 fs 时间尺度以下,解决了先前强场库仑爆炸实验的相互矛盾的发现。我们的综合实验-计算研究表明,质子转移和远程电子转移过程之间的超快竞争决定了漫游的中性 H 2dication 的动力学分别导致\({\mathrm{H}}_3^ +\)或\({\mathrm{H}}_2^ +\)片段。











































 京公网安备 11010802027423号
京公网安备 11010802027423号