Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effect of Intravenous Zoledronic Acid on Tibiofemoral Cartilage Volume Among Patients With Knee Osteoarthritis With Bone Marrow Lesions
JAMA ( IF 63.1 ) Pub Date : 2020-04-21 , DOI: 10.1001/jama.2020.2938 Guoqi Cai 1 , Dawn Aitken 1 , Laura L Laslett 1 , Jean-Pierre Pelletier 2 , Johanne Martel-Pelletier 2 , Catherine Hill 3 , Lyn March 4 , Anita E Wluka 5 , Yuanyuan Wang 5 , Benny Antony 1 , Leigh Blizzard 1 , Tania Winzenberg 1 , Flavia Cicuttini 5 , Graeme Jones 1
JAMA ( IF 63.1 ) Pub Date : 2020-04-21 , DOI: 10.1001/jama.2020.2938 Guoqi Cai 1 , Dawn Aitken 1 , Laura L Laslett 1 , Jean-Pierre Pelletier 2 , Johanne Martel-Pelletier 2 , Catherine Hill 3 , Lyn March 4 , Anita E Wluka 5 , Yuanyuan Wang 5 , Benny Antony 1 , Leigh Blizzard 1 , Tania Winzenberg 1 , Flavia Cicuttini 5 , Graeme Jones 1
Affiliation
|
Importance
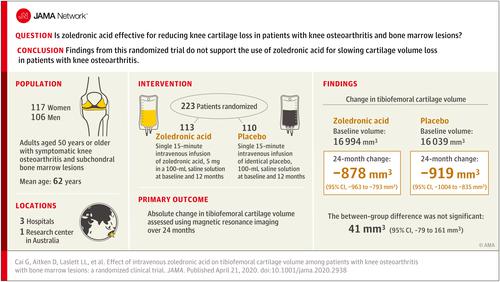
A proof-of-principle study suggested that intravenous zoledronic acid may reduce knee pain and the size of bone marrow lesions in people with knee osteoarthritis, but data from large trials are lacking. Objective
To determine the effects of intravenous zoledronic acid on knee cartilage volume loss in patients with symptomatic knee osteoarthritis and bone marrow lesions. Design, Setting, and Participants
A 24-month multicenter, double-blind placebo-controlled randomized clinical trial conducted at 4 sites in Australia (1 research center and 3 hospitals). Adults aged 50 years or older with symptomatic knee osteoarthritis and subchondral bone marrow lesions detected by magnetic resonance imaging (MRI) were enrolled from November 2013 through September 2015. The final date of follow-up was October 9, 2017. Interventions
Intravenous infusion with either 5 mg of zoledronic acid in a 100-mL saline solution (n = 113) or a placebo saline solution (n = 110) at baseline and 12 months. Main Outcomes and Measures
The primary outcome was absolute change in tibiofemoral cartilage volume assessed using MRI over 24 months (the minimum clinically important difference [MCID] has not been established). Three prespecified secondary outcomes were change in knee pain assessed by a visual analog scale (0 [no pain] to 100 [unbearable pain]; MCID, 15) and the Western Ontario and McMaster Universities Osteoarthritis Index (0 [no pain] to 500 [unbearable pain]; MCID, 75) over 3, 6, 12, 18, and 24 months and change in bone marrow lesion size over 6 and 24 months (the MCID has not been established). Results
Of 223 participants enrolled (mean age, 62.0 years [SD, 8.0 years]; 52% were female), 190 (85%) completed the trial. Change in tibiofemoral cartilage volume was not significantly different between the zoledronic acid group and the placebo group over 24 months (-878 mm3 vs -919 mm3; between-group difference, 41 mm3 [95% CI, -79 to 161 mm3]; P = .50). No significant between-group differences were found for any of the prespecified secondary outcomes, including changes in knee pain assessed by a visual analog scale (-11.5 in the zoledronic acid group vs -16.8 in the placebo group; between-group difference, 5.2 [95% CI, -2.3 to 12.8]; P = .17), changes in knee pain assessed by the Western Ontario and McMaster Universities Osteoarthritis Index (-37.5 vs -58.0, respectively; between-group difference, 20.5 [95% CI, -11.2 to 52.2]; P = .21), and changes in bone marrow lesion size (-33 mm2 vs -6 mm2; between-group difference, -27 mm2 [95% CI, -127 to 73 mm2]; P = .60) over 24 months. Adverse events were more common with zoledronic acid than with placebo (96% vs 83%, respectively) and consisted mainly of acute reactions (defined as symptoms within 3 days of administration of infusion; 87% vs 56%). Conclusions and Relevance
Among patients with symptomatic knee osteoarthritis and bone marrow lesions, yearly zoledronic acid infusions, compared with placebo, did not significantly reduce cartilage volume loss over 24 months. These findings do not support the use of zoledronic acid in the treatment of knee osteoarthritis. Trial Registration
anzctr.org.au Identifier: ACTRN12613000039785.
中文翻译:

静脉注射唑来膦酸对膝骨关节炎合并骨髓病变患者胫骨软骨体积的影响
重要性 一项原理验证研究表明,静脉注射唑来膦酸可能会减轻膝关节骨关节炎患者的膝关节疼痛和骨髓病变的大小,但缺乏来自大型试验的数据。目的探讨静脉注射唑来膦酸对症状性膝骨关节炎合并骨髓病变患者膝关节软骨体积丢失的影响。设计、设置和参与者 一项为期 24 个月的多中心、双盲、安慰剂对照随机临床试验,在澳大利亚的 4 个地点(1 个研究中心和 3 家医院)进行。2013 年 11 月至 2015 年 9 月招募了 50 岁或以上患有症状性膝关节骨关节炎和软骨下骨髓病变的成年人,这些患者通过磁共振成像 (MRI) 检测到。最后的随访日期为 2017 年 10 月 9 日。干预 在基线和 12 个月时静脉输注 5 mg 唑来膦酸的 100 mL 盐水溶液(n = 113)或安慰剂盐溶液(n = 110)。主要结果和测量主要结果是使用 MRI 评估的胫股软骨体积在 24 个月内的绝对变化(最小临床重要差异 [MCID] 尚未确定)。三个预先指定的次要结果是通过视觉模拟量表(0 [无疼痛] 至 100 [无法忍受的疼痛];MCID,15)和西安大略和麦克马斯特大学骨关节炎指数(0 [无疼痛] 至 500 [疼痛])评估膝关节疼痛的变化。无法忍受的疼痛];MCID,75)超过 3、6、12、18 和 24 个月,以及在 6 和 24 个月内骨髓病变大小的变化(MCID 尚未确定)。结果 纳入 223 名参与者(平均年龄,62.0 岁 [SD,8.0 岁];52% 为女性),190 人(85%)完成了试验。24 个月内唑来膦酸组和安慰剂组之间胫股软骨体积的变化无显着差异(-878 mm3 对 -919 mm3;组间差异,41 mm3 [95% CI,-79 至 161 mm3];P = .50)。没有发现任何预先指定的次要结果的显着组间差异,包括通过视觉模拟量表评估的膝关节疼痛的变化(唑来膦酸组为 -11.5,安慰剂组为 -16.8;组间差异为 5.2 [ 95% CI,-2.3 至 12.8];P = .17),西安大略大学和麦克马斯特大学骨关节炎指数评估的膝关节疼痛变化(分别为 -37.5 和 -58.0;组间差异,20.5 [95% CI, -11.2 至 52.2];P = .21),以及骨髓病变大小的变化(-33 mm2 对 -6 mm2;组间差异,-27 mm2 [95% CI,-127 至 73 mm2];P = .60) 超过 24 个月。唑来膦酸的不良事件比安慰剂更常见(分别为 96% 和 83%),主要包括急性反应(定义为输注后 3 天内的症状;87% 对 56%)。结论和相关性 在有症状的膝关节骨关节炎和骨髓病变患者中,与安慰剂相比,每年输注唑来膦酸在 24 个月内没有显着减少软骨体积损失。这些发现不支持使用唑来膦酸治疗膝骨关节炎。试用注册 anzctr.org.au 标识符:ACTRN12613000039785。分别)和主要由急性反应组成(定义为输注后 3 天内的症状;87% 对 56%)。结论和相关性 在有症状的膝关节骨关节炎和骨髓病变患者中,与安慰剂相比,每年输注唑来膦酸在 24 个月内没有显着减少软骨体积损失。这些发现不支持使用唑来膦酸治疗膝骨关节炎。试用注册 anzctr.org.au 标识符:ACTRN12613000039785。分别)和主要由急性反应组成(定义为输注后 3 天内的症状;87% 对 56%)。结论和相关性 在有症状的膝关节骨关节炎和骨髓病变患者中,与安慰剂相比,每年输注唑来膦酸在 24 个月内没有显着减少软骨体积损失。这些发现不支持使用唑来膦酸治疗膝骨关节炎。试用注册 anzctr.org.au 标识符:ACTRN12613000039785。在 24 个月内没有显着减少软骨体积损失。这些发现不支持使用唑来膦酸治疗膝骨关节炎。试用注册 anzctr.org.au 标识符:ACTRN12613000039785。在 24 个月内没有显着减少软骨体积损失。这些发现不支持使用唑来膦酸治疗膝骨关节炎。试用注册 anzctr.org.au 标识符:ACTRN12613000039785。
更新日期:2020-04-21
中文翻译:

静脉注射唑来膦酸对膝骨关节炎合并骨髓病变患者胫骨软骨体积的影响
重要性 一项原理验证研究表明,静脉注射唑来膦酸可能会减轻膝关节骨关节炎患者的膝关节疼痛和骨髓病变的大小,但缺乏来自大型试验的数据。目的探讨静脉注射唑来膦酸对症状性膝骨关节炎合并骨髓病变患者膝关节软骨体积丢失的影响。设计、设置和参与者 一项为期 24 个月的多中心、双盲、安慰剂对照随机临床试验,在澳大利亚的 4 个地点(1 个研究中心和 3 家医院)进行。2013 年 11 月至 2015 年 9 月招募了 50 岁或以上患有症状性膝关节骨关节炎和软骨下骨髓病变的成年人,这些患者通过磁共振成像 (MRI) 检测到。最后的随访日期为 2017 年 10 月 9 日。干预 在基线和 12 个月时静脉输注 5 mg 唑来膦酸的 100 mL 盐水溶液(n = 113)或安慰剂盐溶液(n = 110)。主要结果和测量主要结果是使用 MRI 评估的胫股软骨体积在 24 个月内的绝对变化(最小临床重要差异 [MCID] 尚未确定)。三个预先指定的次要结果是通过视觉模拟量表(0 [无疼痛] 至 100 [无法忍受的疼痛];MCID,15)和西安大略和麦克马斯特大学骨关节炎指数(0 [无疼痛] 至 500 [疼痛])评估膝关节疼痛的变化。无法忍受的疼痛];MCID,75)超过 3、6、12、18 和 24 个月,以及在 6 和 24 个月内骨髓病变大小的变化(MCID 尚未确定)。结果 纳入 223 名参与者(平均年龄,62.0 岁 [SD,8.0 岁];52% 为女性),190 人(85%)完成了试验。24 个月内唑来膦酸组和安慰剂组之间胫股软骨体积的变化无显着差异(-878 mm3 对 -919 mm3;组间差异,41 mm3 [95% CI,-79 至 161 mm3];P = .50)。没有发现任何预先指定的次要结果的显着组间差异,包括通过视觉模拟量表评估的膝关节疼痛的变化(唑来膦酸组为 -11.5,安慰剂组为 -16.8;组间差异为 5.2 [ 95% CI,-2.3 至 12.8];P = .17),西安大略大学和麦克马斯特大学骨关节炎指数评估的膝关节疼痛变化(分别为 -37.5 和 -58.0;组间差异,20.5 [95% CI, -11.2 至 52.2];P = .21),以及骨髓病变大小的变化(-33 mm2 对 -6 mm2;组间差异,-27 mm2 [95% CI,-127 至 73 mm2];P = .60) 超过 24 个月。唑来膦酸的不良事件比安慰剂更常见(分别为 96% 和 83%),主要包括急性反应(定义为输注后 3 天内的症状;87% 对 56%)。结论和相关性 在有症状的膝关节骨关节炎和骨髓病变患者中,与安慰剂相比,每年输注唑来膦酸在 24 个月内没有显着减少软骨体积损失。这些发现不支持使用唑来膦酸治疗膝骨关节炎。试用注册 anzctr.org.au 标识符:ACTRN12613000039785。分别)和主要由急性反应组成(定义为输注后 3 天内的症状;87% 对 56%)。结论和相关性 在有症状的膝关节骨关节炎和骨髓病变患者中,与安慰剂相比,每年输注唑来膦酸在 24 个月内没有显着减少软骨体积损失。这些发现不支持使用唑来膦酸治疗膝骨关节炎。试用注册 anzctr.org.au 标识符:ACTRN12613000039785。分别)和主要由急性反应组成(定义为输注后 3 天内的症状;87% 对 56%)。结论和相关性 在有症状的膝关节骨关节炎和骨髓病变患者中,与安慰剂相比,每年输注唑来膦酸在 24 个月内没有显着减少软骨体积损失。这些发现不支持使用唑来膦酸治疗膝骨关节炎。试用注册 anzctr.org.au 标识符:ACTRN12613000039785。在 24 个月内没有显着减少软骨体积损失。这些发现不支持使用唑来膦酸治疗膝骨关节炎。试用注册 anzctr.org.au 标识符:ACTRN12613000039785。在 24 个月内没有显着减少软骨体积损失。这些发现不支持使用唑来膦酸治疗膝骨关节炎。试用注册 anzctr.org.au 标识符:ACTRN12613000039785。











































 京公网安备 11010802027423号
京公网安备 11010802027423号