当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of Cryptic Largimycins in Streptomyces Reveals Novel Biosynthetic Avenues Enriching the Structural Diversity of the Leinamycin Family.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-04-20 , DOI: 10.1021/acschembio.0c00160 Adriana Becerril 1, 2 , Ignacio Pérez-Victoria 3 , Suhui Ye 1, 2 , Alfredo F Braña 1, 2 , Jesús Martín 3 , Fernando Reyes 3 , José A Salas 1, 2 , Carmen Méndez 1, 2
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-04-20 , DOI: 10.1021/acschembio.0c00160 Adriana Becerril 1, 2 , Ignacio Pérez-Victoria 3 , Suhui Ye 1, 2 , Alfredo F Braña 1, 2 , Jesús Martín 3 , Fernando Reyes 3 , José A Salas 1, 2 , Carmen Méndez 1, 2
Affiliation
|
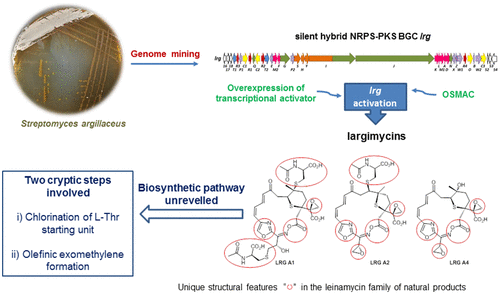
Largimycins are hybrid nonribosomal peptide-polyketides that constitute a new group of metabolites in the leinamycin family of natural products displaying unique structural features such as containing an oxazole instead of a thiazole ring or being oxime ester macrocycles, unprecedented in nature, rather than macrolactams. Their discovery in Streptomyces argillaceus and Streptomyces canus has relied on the activation of two homologous silent gene clusters by overexpressing a transcriptional activator and cultivating in specific media. The proposed biosynthesis of largimycins includes the key action of the oxidoreductase LrgO, responsible for the formation of the oxime group involved in macrocyclization, and two putative cryptic biosynthetic steps consisting of chlorination of l-Thr by the NRPS loading module and incorporation of an olefinic exomethylene group by LrgJ PKS. The discovery of largimycins uncovers novel biosynthetic avenues employed in nature to enrich the structural diversity of leinamycins and provides tools for combinatorial biosynthesis.
中文翻译:

链霉菌中隐性霉素的发现揭示了丰富的莱那霉素家族结构多样性的新型生物合成途径。
拉吉霉素是杂合的非核糖体肽-聚酮化合物,构成天然产品莱纳霉素家族中的一组新的代谢产物,表现出独特的结构特征,例如含有恶唑而不是噻唑环或为肟酯大环,这是自然界前所未有的,而不是大内酰胺。他们在精链霉菌和链霉菌中的发现依赖于两个同源沉默基因簇的激活,这是通过过度表达转录激活因子并在特定培养基中培养而实现的。拟议的拉格霉素的生物合成包括氧化还原酶LrgO的关键作用(负责参与大环化的肟基团的形成),以及两个假定的隐秘生物合成步骤,包括氯化三氯甲烷。NRPS装载模块将1- Thr引入,LrgJ PKS引入烯属环己基。拉格霉素的发现揭示了自然界中用于丰富莱纳霉素结构多样性的新型生物合成途径,并提供了组合生物合成的工具。
更新日期:2020-06-19
中文翻译:

链霉菌中隐性霉素的发现揭示了丰富的莱那霉素家族结构多样性的新型生物合成途径。
拉吉霉素是杂合的非核糖体肽-聚酮化合物,构成天然产品莱纳霉素家族中的一组新的代谢产物,表现出独特的结构特征,例如含有恶唑而不是噻唑环或为肟酯大环,这是自然界前所未有的,而不是大内酰胺。他们在精链霉菌和链霉菌中的发现依赖于两个同源沉默基因簇的激活,这是通过过度表达转录激活因子并在特定培养基中培养而实现的。拟议的拉格霉素的生物合成包括氧化还原酶LrgO的关键作用(负责参与大环化的肟基团的形成),以及两个假定的隐秘生物合成步骤,包括氯化三氯甲烷。NRPS装载模块将1- Thr引入,LrgJ PKS引入烯属环己基。拉格霉素的发现揭示了自然界中用于丰富莱纳霉素结构多样性的新型生物合成途径,并提供了组合生物合成的工具。











































 京公网安备 11010802027423号
京公网安备 11010802027423号