当前位置:
X-MOL 学术
›
Stem Cells Transl. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preclinical safety studies of human embryonic stem cell-derived retinal pigment epithelial cells for the treatment of age-related macular degeneration.
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2020-04-22 , DOI: 10.1002/sctm.19-0396 Sandra Petrus-Reurer 1, 2, 3, 4 , Pankaj Kumar 1, 2, 3 , Sara Padrell Sánchez 1, 2, 3 , Monica Aronsson 4 , Helder André 4 , Hammurabi Bartuma 4 , Alvaro Plaza Reyes 1, 2, 3 , Emeline F Nandrot 5 , Anders Kvanta 4 , Fredrik Lanner 1, 2, 3
STEM CELLS Translational Medicine ( IF 5.4 ) Pub Date : 2020-04-22 , DOI: 10.1002/sctm.19-0396 Sandra Petrus-Reurer 1, 2, 3, 4 , Pankaj Kumar 1, 2, 3 , Sara Padrell Sánchez 1, 2, 3 , Monica Aronsson 4 , Helder André 4 , Hammurabi Bartuma 4 , Alvaro Plaza Reyes 1, 2, 3 , Emeline F Nandrot 5 , Anders Kvanta 4 , Fredrik Lanner 1, 2, 3
Affiliation
|
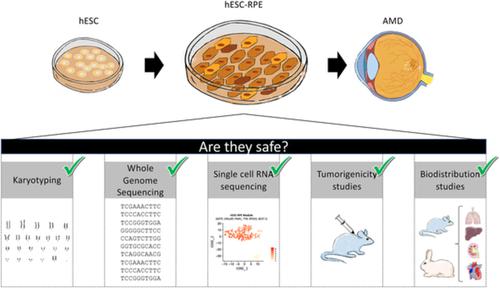
As pluripotent stem cell (PSC)‐based reparative cell therapies are reaching the bedside, there is a growing need for the standardization of studies concerning safety of the derived products. Clinical trials using these promising strategies are in development, and treatment for age‐related macular degeneration is one of the first that has reached patients. We have previously established a xeno‐free and defined differentiation protocol to generate functional human embryonic stem cells (hESCs)‐derived retinal pigment epithelial (RPE) cells. In this study, we perform preclinical safety studies including karyotype and whole‐genome sequencing (WGS) to assess genome stability, single‐cell RNA sequencing to ensure cell purity, and biodistribution and tumorigenicity analysis to rule out potential migratory or tumorigenic properties of these cells. WGS analysis illustrates that existing germline variants load is higher than the introduced variants acquired through in vitro culture or differentiation, and enforces the importance to examine the genome integrity at a deeper level than just karyotype. Altogether, we provide a strategy for preclinical evaluation of PSC‐based therapies and the data support safety of the hESC‐RPE cells generated through our in vitro differentiation methodology.
中文翻译:

人类胚胎干细胞来源的视网膜色素上皮细胞治疗年龄相关性黄斑变性的临床前安全性研究。
随着基于多能干细胞(PSC)的修复性细胞疗法在床边普及,对衍生产品安全性研究标准化的需求日益增长。使用这些有前途的策略的临床试验正在开发中,与年龄有关的黄斑变性的治疗是最早进入患者的治疗方法之一。我们之前已经建立了无异种的定义分化方案,以生成功能性人类胚胎干细胞(hESCs)衍生的视网膜色素上皮细胞(RPE)。在这项研究中,我们进行临床前安全性研究,包括核型和全基因组测序(WGS)评估基因组稳定性,单细胞RNA测序以确保细胞纯度,以及生物分布和致瘤性分析以排除这些细胞的潜在迁移或致癌特性。WGS分析表明,现有种系变异体的负载量高于通过体外培养或分化获得的引入变异体,并加强了在不仅仅是核型的更深层次上检查基因组完整性的重要性。总之,我们提供了一种基于PSC疗法的临床前评估策略以及通过我们的体外分化方法生成的hESC-RPE细胞的数据支持安全性的策略。
更新日期:2020-04-22
中文翻译:

人类胚胎干细胞来源的视网膜色素上皮细胞治疗年龄相关性黄斑变性的临床前安全性研究。
随着基于多能干细胞(PSC)的修复性细胞疗法在床边普及,对衍生产品安全性研究标准化的需求日益增长。使用这些有前途的策略的临床试验正在开发中,与年龄有关的黄斑变性的治疗是最早进入患者的治疗方法之一。我们之前已经建立了无异种的定义分化方案,以生成功能性人类胚胎干细胞(hESCs)衍生的视网膜色素上皮细胞(RPE)。在这项研究中,我们进行临床前安全性研究,包括核型和全基因组测序(WGS)评估基因组稳定性,单细胞RNA测序以确保细胞纯度,以及生物分布和致瘤性分析以排除这些细胞的潜在迁移或致癌特性。WGS分析表明,现有种系变异体的负载量高于通过体外培养或分化获得的引入变异体,并加强了在不仅仅是核型的更深层次上检查基因组完整性的重要性。总之,我们提供了一种基于PSC疗法的临床前评估策略以及通过我们的体外分化方法生成的hESC-RPE细胞的数据支持安全性的策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号