当前位置:
X-MOL 学术
›
Part. Part. Syst. Charact.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Brownmillerite‐Type Crystalline Ca2FeCoO5 Ultrasmall Particles with Single‐Nanometer Dimensions as an Active Cocatalyst for Oxygen Photoevolution Reaction
Particle & Particle Systems Characterization ( IF 2.7 ) Pub Date : 2020-04-20 , DOI: 10.1002/ppsc.202000053 Etsushi Tsuji 1 , Ryosuke Nanbu 1 , Yoshiki Degami 1 , Kei Hirao 1 , Takeyuki Watanabe 1 , Naoya Matsumoto 1 , Satoshi Suganuma 1 , Naonobu Katada 1
Particle & Particle Systems Characterization ( IF 2.7 ) Pub Date : 2020-04-20 , DOI: 10.1002/ppsc.202000053 Etsushi Tsuji 1 , Ryosuke Nanbu 1 , Yoshiki Degami 1 , Kei Hirao 1 , Takeyuki Watanabe 1 , Naoya Matsumoto 1 , Satoshi Suganuma 1 , Naonobu Katada 1
Affiliation
|
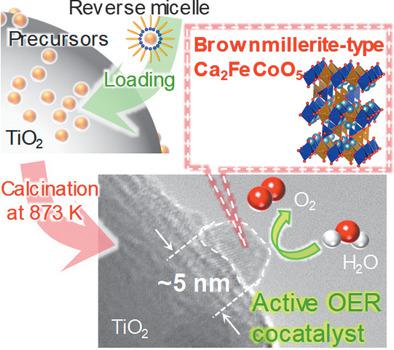
Brownmillerite‐type Ca2FeCoO5 (CFCO) is one of the most effective catalysts for oxygen evolution reaction (OER), comparable with noble metal oxides. In this study, crystalline CFCO ultrasmall particles with nanometric dimension are synthesized by a reverse micelle method on TiO2 nanoparticles. The particle size decreases with decreasing molar ratio of water to surfactant. The precursors of CFCO must be calcined after loading on TiO2 nanoparticles to achieve CFCO ultrasmall particles with several nanometers in size. Interaction between the precursors and TiO2 is speculated to suppress aggregation of the precursors during calcination. The photocatalytic activity of TiO2 for OER is improved by loading of CFCO ultrasmall particles with 5 nm, whereas the activity decreases by loading of CFCO with more than 15 nm. Photocatalytic activity of the most active CFCO/TiO2 is comparable to that of RuO2/TiO2. Both the lower edge of conduction band and higher edge of valence band of CFCO are lower and higher than those of TiO2, respectively, leading to transfer of excited holes and electrons transferred to CFCO and recombination. When the particle size of CFCO becomes several nanometric dimensions, the transferred holes rapidly reach the surface of CFCO and oxidize water molecules before recombination with electrons.
中文翻译:

具有纳米级尺寸的棕刚玉型结晶Ca2FeCoO5超细颗粒作为氧气光进化反应的活性助催化剂
与贵金属氧化物相比,褐煤型Ca 2 FeCoO 5(CFCO )是最有效的氧释放反应(OER)催化剂之一。在这项研究中,通过反胶束法在TiO 2纳米粒子上合成了纳米级的CFCO纳米晶体。粒度随着水与表面活性剂的摩尔比降低而减小。负载在TiO 2纳米颗粒上后,必须煅烧CFCO的前驱体,以获得尺寸为几纳米的CFCO超小颗粒。推测前体和TiO 2之间的相互作用可抑制煅烧期间前体的聚集。TiO 2的光催化活性通过装载5 nm的CFCO超细颗粒可以改善OER的活性,而装载超过15 nm的CFCO可以降低活性。活性最高的CFCO / TiO 2的光催化活性与RuO 2 / TiO 2相当。CFCO的导带的下边缘和价带的上边缘分别比TiO 2的低和高,导致激发空穴的转移和电子转移到CFCO并发生复合。当CFCO的粒径变为数个纳米尺寸时,转移的空穴迅速到达CFCO的表面并氧化水分子,然后再与电子复合。
更新日期:2020-04-20
中文翻译:

具有纳米级尺寸的棕刚玉型结晶Ca2FeCoO5超细颗粒作为氧气光进化反应的活性助催化剂
与贵金属氧化物相比,褐煤型Ca 2 FeCoO 5(CFCO )是最有效的氧释放反应(OER)催化剂之一。在这项研究中,通过反胶束法在TiO 2纳米粒子上合成了纳米级的CFCO纳米晶体。粒度随着水与表面活性剂的摩尔比降低而减小。负载在TiO 2纳米颗粒上后,必须煅烧CFCO的前驱体,以获得尺寸为几纳米的CFCO超小颗粒。推测前体和TiO 2之间的相互作用可抑制煅烧期间前体的聚集。TiO 2的光催化活性通过装载5 nm的CFCO超细颗粒可以改善OER的活性,而装载超过15 nm的CFCO可以降低活性。活性最高的CFCO / TiO 2的光催化活性与RuO 2 / TiO 2相当。CFCO的导带的下边缘和价带的上边缘分别比TiO 2的低和高,导致激发空穴的转移和电子转移到CFCO并发生复合。当CFCO的粒径变为数个纳米尺寸时,转移的空穴迅速到达CFCO的表面并氧化水分子,然后再与电子复合。



























 京公网安备 11010802027423号
京公网安备 11010802027423号