当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An autoinhibitory mechanism controls RNA-binding activity of the nitrate-sensing protein NasR.
Molecular Microbiology ( IF 2.6 ) Pub Date : 2020-04-20 , DOI: 10.1111/mmi.14517 Jonathan R Goodson 1 , Christopher Zhang 1 , Daniel Trettel 2 , Heather E Ailinger 3 , Priscilla E Lee 3 , Catherine M Spirito 3 , Wade C Winkler 1, 2, 3
Molecular Microbiology ( IF 2.6 ) Pub Date : 2020-04-20 , DOI: 10.1111/mmi.14517 Jonathan R Goodson 1 , Christopher Zhang 1 , Daniel Trettel 2 , Heather E Ailinger 3 , Priscilla E Lee 3 , Catherine M Spirito 3 , Wade C Winkler 1, 2, 3
Affiliation
|
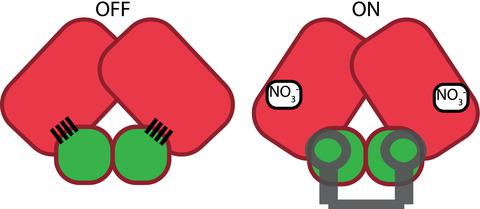
The ANTAR domain harnesses RNA‐binding activity to promote transcription attenuation. Although several ANTAR proteins have been analyzed by high‐resolution structural analyses, the residues involved in RNA‐recognition and transcription attenuation have not been identified. Nor is it clear how signal‐responsive domains are allosterically coupled with ANTAR domains for control of gene expression. Herein, we examined the sequence conservation of ANTAR domains to find residues that may associate with RNA. We subjected the corresponding positions of Klebsiella oxytoca NasR to site‐directed alanine substitutions and measured RNA‐binding activity. This revealed a functionally important patch of residues that forms amino acid pairing interactions with residues from NasR’s nitrate‐sensing NIT domain. We hypothesize these amino acid pairing interactions are part of an autoinhibitory mechanism that holds the structure in an “off” state in the absence of nitrate signal. Indeed, mutational disruption of these interactions resulted in constitutively active proteins, freed from autoinhibition and no longer influenced by nitrate. Moreover, sequence analyses suggested the autoinhibitory mechanism has been evolutionarily maintained by NasR proteins. These data reveal a molecular mechanism for how NasR couples its nitrate signal to RNA‐binding activity, and generally show how signal‐responsive domains of one‐component regulatory proteins have evolved to exert control over RNA‐binding ANTAR domains.
中文翻译:

一种自抑制机制控制硝酸盐敏感蛋白NasR的RNA结合活性。
ANTAR域利用RNA结合活性来促进转录衰减。尽管已经通过高分辨率结构分析对几种ANTAR蛋白进行了分析,但尚未鉴定出与RNA识别和转录衰减有关的残基。还不清楚如何将信号响应域与ANTAR域进行变构偶联以控制基因表达。在这里,我们检查了ANTAR域的序列保守性,以发现可能与RNA相关的残基。我们对产酸克雷伯菌进行了相应定位NasR定位丙氨酸取代和测量RNA结合活性。这揭示了一个重要的残基补丁,该残基与NasR的硝酸盐敏感NIT域中的残基形成氨基酸配对相互作用。我们假设这些氨基酸配对相互作用是自动抑制机制的一部分,该机制在硝酸盐信号不存在的情况下将结构保持在“关闭”状态。确实,这些相互作用的突变破坏导致组成型活性蛋白脱离了自抑制作用,并且不再受硝酸盐的影响。此外,序列分析表明,NasR蛋白已经进化地维持了自身抑制机制。这些数据揭示了NasR如何将其硝酸盐信号与RNA结合活性偶联的分子机制,
更新日期:2020-04-20
中文翻译:

一种自抑制机制控制硝酸盐敏感蛋白NasR的RNA结合活性。
ANTAR域利用RNA结合活性来促进转录衰减。尽管已经通过高分辨率结构分析对几种ANTAR蛋白进行了分析,但尚未鉴定出与RNA识别和转录衰减有关的残基。还不清楚如何将信号响应域与ANTAR域进行变构偶联以控制基因表达。在这里,我们检查了ANTAR域的序列保守性,以发现可能与RNA相关的残基。我们对产酸克雷伯菌进行了相应定位NasR定位丙氨酸取代和测量RNA结合活性。这揭示了一个重要的残基补丁,该残基与NasR的硝酸盐敏感NIT域中的残基形成氨基酸配对相互作用。我们假设这些氨基酸配对相互作用是自动抑制机制的一部分,该机制在硝酸盐信号不存在的情况下将结构保持在“关闭”状态。确实,这些相互作用的突变破坏导致组成型活性蛋白脱离了自抑制作用,并且不再受硝酸盐的影响。此外,序列分析表明,NasR蛋白已经进化地维持了自身抑制机制。这些数据揭示了NasR如何将其硝酸盐信号与RNA结合活性偶联的分子机制,









































 京公网安备 11010802027423号
京公网安备 11010802027423号