Structure ( IF 4.4 ) Pub Date : 2020-04-21 , DOI: 10.1016/j.str.2020.03.012 Nathanael A Caveney 1 , Alexander J F Egan 2 , Isabel Ayala 3 , Cédric Laguri 3 , Craig S Robb 1 , Eefjan Breukink 4 , Waldemar Vollmer 2 , Natalie C J Strynadka 1 , Jean-Pierre Simorre 3
|
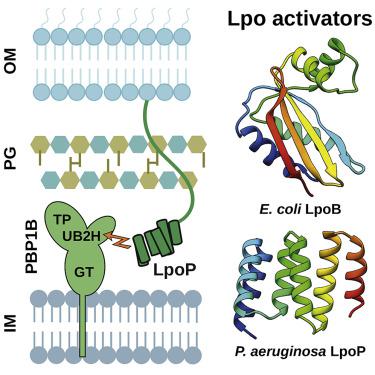
Peptidoglycan (PG) is an essential component of the bacterial cell wall and is assembled from a lipid II precursor by glycosyltransferase and transpeptidase reactions catalyzed in particular by bifunctional class A penicillin-binding proteins (aPBPs). In the major clinical pathogen Pseudomonas aeruginosa, PBP1B is anchored within the cytoplasmic membrane but regulated by a bespoke outer membrane-localized lipoprotein known as LpoP. Here, we report the structure of LpoP, showing an extended N-terminal, flexible tether followed by a well-ordered C-terminal tandem-tetratricopeptide repeat domain. We show that LpoP stimulates both PBP1B transpeptidase and glycosyltransferase activities in vitro and interacts directly via its C terminus globular domain with the central UB2H domain of PBP1B. Contrary to the situation in E. coli, P. aeruginosa CpoB does not regulate PBP1B/LpoP in vitro. We propose a mechanism that helps to underscore similarities and differences in class A PBP activation across Gram-negative bacteria.
中文翻译:

铜绿假单胞菌肽聚糖合酶激活剂 LpoP 的结构。
肽聚糖 (PG) 是细菌细胞壁的重要组成部分,由脂质 II 前体通过糖基转移酶和转肽酶反应组装而成,特别是由双功能 A 类青霉素结合蛋白 (aPBP) 催化。在主要的临床病原体铜绿假单胞菌中,PBP1B 锚定在细胞质膜内,但受到称为 LpoP 的定制外膜定位脂蛋白的调节。在这里,我们报告了 LpoP 的结构,显示了一个延伸的 N 端柔性系链,后面是一个有序的 C 端串联四肽重复结构域。我们发现,LpoP在体外刺激 PBP1B 转肽酶和糖基转移酶活性,并通过其 C 末端球状结构域与 PBP1B 的中央 UB2H 结构域直接相互作用。与大肠杆菌的情况相反,铜绿假单胞菌CpoB在体外不调节PBP1B/LpoP。我们提出了一种机制,有助于强调革兰氏阴性细菌 A 类 PBP 激活的相似性和差异。









































 京公网安备 11010802027423号
京公网安备 11010802027423号