Structure ( IF 4.4 ) Pub Date : 2020-04-21 , DOI: 10.1016/j.str.2020.03.014 Burçin Acar 1 , Jessica Rose 2 , Burcu Aykac Fas 1 , Nir Ben-Tal 3 , Oded Lewinson 2 , Turkan Haliloglu 1
|
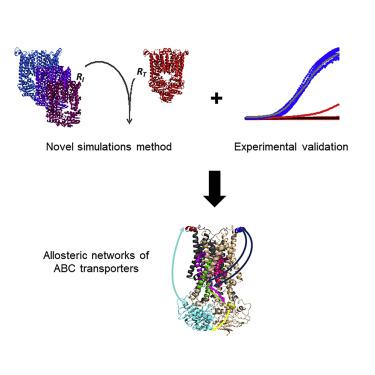
ABC transporters couple the energy of ATP hydrolysis to the transmembrane transport of biomolecules. Here, we investigated the allosteric networks of three representative ABC transporters using a hybrid molecular simulations approach validated by experiments. Each of the three transporters uses a different allosteric network: in the constitutive B12 importer BtuCD, ATP binding is the main driver of allostery and docking/undocking of the substrate-binding protein (SBP) is the driven event. The allosteric signal originates at the cytoplasmic side of the membrane before propagating to the extracellular side. In the substrate-controlled maltose transporter, the SBP is the main driver of allostery, ATP binding is the driven event, and the allosteric signal propagates from the extracellular to the cytoplasmic side of the membrane. In the lipid flippase PglK, a cyclic crosstalk between ATP and substrate binding underlies allostery. These results demonstrate speciation of biological functions may arise from variations in allosteric connectivity.
中文翻译:

不同的变构网络是ABC转运蛋白的机械形态的基础。
ABC转运蛋白将ATP水解的能量与生物分子的跨膜转运耦合。在这里,我们使用经过实验验证的混合分子模拟方法研究了三个代表性ABC转运蛋白的变构网络。这三个转运蛋白各自使用不同的变构网络:在组成型B12进口商BtuCD中,ATP结合是变构的主要驱动力,底物结合蛋白(SBP)的对接/对接是驱动事件。变构信号起源于膜的细胞质侧,然后传播到细胞外侧。在受底物控制的麦芽糖转运蛋白中,SBP是变构的主要驱动力,ATP结合是驱动的事件,变构信号从细胞外传播到膜的细胞质侧。在脂质脂酶PglK中,ATP和底物结合之间的循环串扰是变构的基础。这些结果表明,生物学功能的形成可能是由于变构连接性的变化而引起的。










































 京公网安备 11010802027423号
京公网安备 11010802027423号