Current Organic Synthesis ( IF 1.7 ) Pub Date : 2020-07-31 , DOI: 10.2174/1570179417666200415152105 Duong Ngoc Toan 1 , Nguyen Dinh Thanh 2 , Mai Xuan Truong 1 , Nguyen Minh Thao 2
|
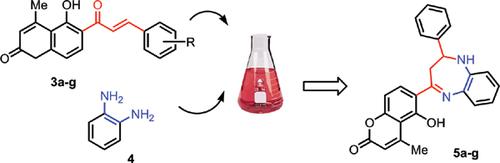
Background: Reaction of α,β-unsaturated ketones with o-phenylenediamine afforded corresponding 2,3-dihydro-1H-1,5-benzodiazepines.
Objective: α,β-Unsaturated ketones of 6-acetyl-5-hydroxy-4-methylcoumarin are precursors for synthesis of 2,3-dihydro-1H-1,5-benzodiazepines by a reaction with o-phenylenediamine.
Methods: Enones of 6-acetyl-5-hydroxy-4-methylcoumarin were prepared from this ketone and (un)substituted benzaldehydes in the presence of piperidine, triethylamine, or pyridine as a catalyst in absolute ethanol with 1:1 molar ratios, respectively. 2',3'-Dihydro-1H-1',5'-benzodiazepines were synthesized by using the reaction of these enones with o-phenylenediamine in absolute ethanol in the presence of glacial acetic acid as a catalyst. Their biological activities were evaluated using the disk diffusion method.
Results: Seven new 2',3'-dihydro-1H-1',5'-benzodiazepines were obtained and their structures were confirmed by thin-layer chromatography, IR, NMR and MS spectra. Some synthesized benzodiazepines showed antibacterial and antifungal activities against Escherichia coli (Gram-(−) bacterium), Staphylococus epidermidis (Gram-(+) bacterium). Candida albicans (fungus).
Conclusion: The formation of enones from 6-acetyl-5-hydroxy-4-methylcoumarin and (un)substituted benzaldehydes could be catalyzed by piperidine, triethylamine, pyridine to afford similar yields. 2',3'-dihydro-1H- 1',5'-benzodiazepines have been synthesized from the aforementioned enones and o-phenylenediamine.
中文翻译:

由6-乙酰基-5-羟基-4-甲基香豆素的α,β-不饱和酮合成一些含铬环的1H-1,5-苯并二氮杂Series系列。
背景:α,β-不饱和酮与邻苯二胺反应得到相应的2,3-二氢-1H-1,5-苯并二氮杂pine。
目的:6-乙酰基-5-羟基-4-甲基香豆素的α,β-不饱和酮是通过与邻苯二胺反应合成2,3-二氢-1H-1,5-苯并二氮杂的前体。
方法:在无水乙醇中以哌啶,三乙胺或吡啶为催化剂,分别以1:1的摩尔比,由该酮和(未)取代的苯甲醛制备6-乙酰基-5-羟基-4-甲基香豆素的烯酮。通过将这些烯酮与邻苯二胺在无水乙醇中,在冰醋酸作为催化剂存在下的反应,合成了2',3'-二氢-1H-1',5'-苯并二氮杂synthesized。使用圆盘扩散法评估其生物活性。
结果:获得了七个新的2',3'-二氢-1H-1',5'-苯并二氮杂卓,并通过薄层色谱,IR,NMR和MS光谱确认了其结构。一些合成的苯二氮卓类对大肠杆菌(Gram-(-)细菌),表皮葡萄球菌(Gram-(+)细菌)具有抗菌和抗真菌活性。白色念珠菌(真菌)。
结论:哌啶,三乙胺,吡啶可催化由6-乙酰基-5-羟基-4-甲基香豆素和(未)取代的苯甲醛形成烯酮,产率相似。由上述烯酮和邻苯二胺合成了2′,3′-二氢-1H-1′,5′-苯并二氮杂。











































 京公网安备 11010802027423号
京公网安备 11010802027423号