当前位置:
X-MOL 学术
›
Nat. Struct. Mol. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Conformational plasticity of the ClpAP AAA+ protease couples protein unfolding and proteolysis.
Nature Structural & Molecular Biology ( IF 12.5 ) Pub Date : 2020-04-20 , DOI: 10.1038/s41594-020-0409-5 Kyle E Lopez 1, 2 , Alexandrea N Rizo 2, 3 , Eric Tse 2 , JiaBei Lin 4 , Nathaniel W Scull 5 , Aye C Thwin 2 , Aaron L Lucius 5 , James Shorter 4 , Daniel R Southworth 2
Nature Structural & Molecular Biology ( IF 12.5 ) Pub Date : 2020-04-20 , DOI: 10.1038/s41594-020-0409-5 Kyle E Lopez 1, 2 , Alexandrea N Rizo 2, 3 , Eric Tse 2 , JiaBei Lin 4 , Nathaniel W Scull 5 , Aye C Thwin 2 , Aaron L Lucius 5 , James Shorter 4 , Daniel R Southworth 2
Affiliation
|
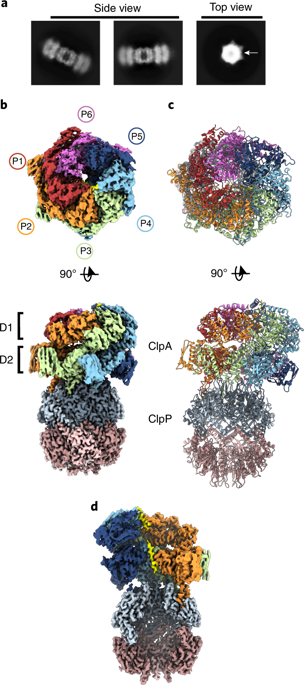
The ClpAP complex is a conserved bacterial protease that unfolds and degrades proteins targeted for destruction. The ClpA double-ring hexamer powers substrate unfolding and translocation into the ClpP proteolytic chamber. Here, we determined high-resolution structures of wild-type Escherichia coli ClpAP undergoing active substrate unfolding and proteolysis. A spiral of pore loop-substrate contacts spans both ClpA AAA+ domains. Protomers at the spiral seam undergo nucleotide-specific rearrangements, supporting substrate translocation. IGL loops extend flexibly to bind the planar, heptameric ClpP surface with the empty, symmetry-mismatched IGL pocket maintained at the seam. Three different structures identify a binding-pocket switch by the IGL loop of the lowest positioned protomer, involving release and re-engagement with the clockwise pocket. This switch is coupled to a ClpA rotation and a network of conformational changes across the seam, suggesting that ClpA can rotate around the ClpP apical surface during processive steps of translocation and proteolysis.
中文翻译:

ClpAP AAA+ 蛋白酶的构象可塑性与蛋白质解折叠和蛋白水解相结合。
ClpAP 复合物是一种保守的细菌蛋白酶,可展开并降解目标蛋白质。 ClpA 双环六聚体为底物展开和易位到 ClpP 蛋白水解室提供动力。在这里,我们确定了野生型大肠杆菌 ClpAP 经历活性底物解折叠和蛋白水解的高分辨率结构。孔环-基底接触的螺旋跨越两个 ClpA AAA+ 结构域。螺旋接缝处的原基经历核苷酸特异性重排,支持底物易位。 IGL 环可灵活延伸,将平面七聚 ClpP 表面与接缝处保留的空的、对称不匹配的 IGL 袋结合在一起。三种不同的结构通过最低位置原聚体的 IGL 环来识别结合袋开关,涉及顺时针袋的释放和重新接合。该开关与 ClpA 旋转和接缝上的构象变化网络耦合,表明 ClpA 在易位和蛋白水解的过程步骤中可以围绕 ClpP 顶端表面旋转。
更新日期:2020-04-24
中文翻译:

ClpAP AAA+ 蛋白酶的构象可塑性与蛋白质解折叠和蛋白水解相结合。
ClpAP 复合物是一种保守的细菌蛋白酶,可展开并降解目标蛋白质。 ClpA 双环六聚体为底物展开和易位到 ClpP 蛋白水解室提供动力。在这里,我们确定了野生型大肠杆菌 ClpAP 经历活性底物解折叠和蛋白水解的高分辨率结构。孔环-基底接触的螺旋跨越两个 ClpA AAA+ 结构域。螺旋接缝处的原基经历核苷酸特异性重排,支持底物易位。 IGL 环可灵活延伸,将平面七聚 ClpP 表面与接缝处保留的空的、对称不匹配的 IGL 袋结合在一起。三种不同的结构通过最低位置原聚体的 IGL 环来识别结合袋开关,涉及顺时针袋的释放和重新接合。该开关与 ClpA 旋转和接缝上的构象变化网络耦合,表明 ClpA 在易位和蛋白水解的过程步骤中可以围绕 ClpP 顶端表面旋转。










































 京公网安备 11010802027423号
京公网安备 11010802027423号