当前位置:
X-MOL 学术
›
Cell Death Differ.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deficiency in fibroblast PPARβ/δ reduces nonmelanoma skin cancers in mice.
Cell Death and Differentiation ( IF 13.7 ) Pub Date : 2020-04-20 , DOI: 10.1038/s41418-020-0535-y Mark Wei Yi Tan 1, 2 , Ming Keat Sng 3 , Hong Sheng Cheng 1, 3 , Zun Siong Low 3 , Benjamin Jia Juin Leong 1 , Damien Chua 1 , Eddie Han Pin Tan 1 , Jeremy Soon Kiat Chan 1 , Yun Sheng Yip 1, 3 , Yin Hao Lee 3 , Mintu Pal 4 , Xiaomeng Wang 3, 5, 6, 7 , Walter Wahli 3, 8, 9 , Nguan Soon Tan 1, 3
Cell Death and Differentiation ( IF 13.7 ) Pub Date : 2020-04-20 , DOI: 10.1038/s41418-020-0535-y Mark Wei Yi Tan 1, 2 , Ming Keat Sng 3 , Hong Sheng Cheng 1, 3 , Zun Siong Low 3 , Benjamin Jia Juin Leong 1 , Damien Chua 1 , Eddie Han Pin Tan 1 , Jeremy Soon Kiat Chan 1 , Yun Sheng Yip 1, 3 , Yin Hao Lee 3 , Mintu Pal 4 , Xiaomeng Wang 3, 5, 6, 7 , Walter Wahli 3, 8, 9 , Nguan Soon Tan 1, 3
Affiliation
|
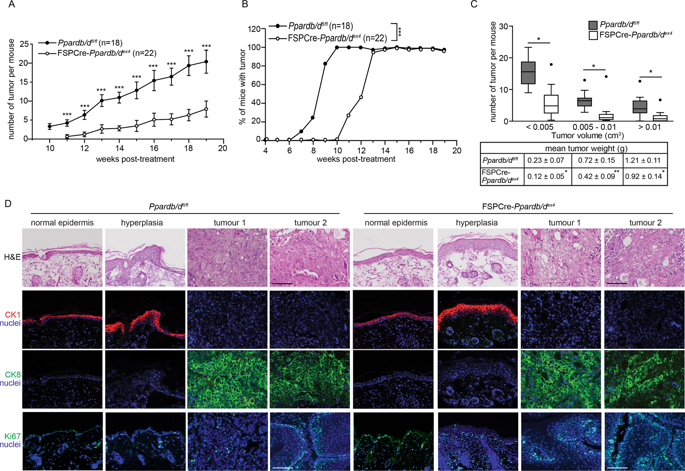
The incidence of nonmelanoma skin cancer (NMSC) has been increasing worldwide. Most studies have highlighted the importance of cancer-associated fibroblasts (CAFs) in NMSC progression. However much less is known about the communication between normal fibroblasts and epithelia; disruption of this communication affects tumor initiation and the latency period in the emergence of tumors. Delineating the mechanism that mediates this epithelial-mesenchymal communication in NMSC could identify more effective targeted therapies. The nuclear receptor PPARβ/δ in fibroblasts has been shown to modulate adjacent epithelial cell behavior, however, its role in skin tumorigenesis remains unknown. Using chemically induced skin carcinogenesis, we showed that FSPCre-Pparb/dex4 mice, whose Pparb/d gene was selectively deleted in fibroblasts, had delayed emergence and reduced tumor burden compared with control mice (Pparb/dfl/fl). However, FSPCre-Pparb/dex4-derived tumors showed increased proliferation, with no difference in differentiation, suggesting delayed tumor initiation. Network analysis revealed a link between dermal Pparb/d and TGF-β1 with epidermal NRF2 and Nox4. In vitro investigations showed that PPARβ/δ deficiency in fibroblasts increased epidermal Nox4-derived H2O2 production, which triggered an NRF2-mediated antioxidant response. We further showed that H2O2 upregulated NRF2 mRNA via the B-Raf-MEK1/2 pathway. The enhanced NRF2 response altered the activities of PTEN, Src, and AKT. In vivo, we detected the differential phosphorylation profiles of B-Raf, MEK1/2, PTEN, Src, and AKT in the vehicle-treated and chemically treated epidermis of FSPCre-Pparb/dex4 mice compared with that in Pparb/dfl/fl mice, prior to the first appearance of tumors in Pparb/dfl/fl. Our study revealed a role for fibroblast PPARβ/δ in the epithelial-mesenchymal communication involved in cellular redox homeostasis.
中文翻译:

成纤维细胞 PPARβ/δ 的缺乏会减少小鼠的非黑色素瘤皮肤癌。
非黑色素瘤皮肤癌 (NMSC) 的发病率在全球范围内呈上升趋势。大多数研究都强调了癌症相关成纤维细胞 (CAF) 在 NMSC 进展中的重要性。然而,对正常成纤维细胞和上皮细胞之间的交流知之甚少。这种通讯的中断会影响肿瘤的发生和肿瘤出现的潜伏期。描述在 NMSC 中介导这种上皮间充质通讯的机制可以确定更有效的靶向治疗。成纤维细胞中的核受体 PPARβ/δ 已被证明可调节邻近的上皮细胞行为,然而,其在皮肤肿瘤发生中的作用仍然未知。使用化学诱导的皮肤癌发生,我们发现 FSPCre-Pparb/dex4 小鼠,其 Pparb/d 基因在成纤维细胞中被选择性删除,与对照小鼠(Pparb / dfl / fl)相比,出现延迟并减少了肿瘤负荷。然而,FSPCre-Pparb/dex4 衍生的肿瘤显示增殖增加,分化没有差异,表明肿瘤起始延迟。网络分析揭示了真皮 Pparb/d 和 TGF-β1 与表皮 NRF2 和 Nox4 之间的联系。体外研究表明,成纤维细胞中 PPARβ/δ 缺乏会增加表皮 Nox4 衍生的 H2O2 产生,从而引发 NRF2 介导的抗氧化反应。我们进一步表明 H2O2 通过 B-Raf-MEK1/2 通路上调 NRF2 mRNA。增强的 NRF2 反应改变了 PTEN、Src 和 AKT 的活性。在体内,我们检测到 B-Raf、MEK1/2、PTEN、Src、与 Pparb/dfl/fl 小鼠相比,在 Pparb/dfl/fl 中首次出现肿瘤之前,FSPCre-Pparb/dex4 小鼠的载体处理和化学处理的表皮中的 AKT 和 AKT。我们的研究揭示了成纤维细胞 PPARβ/δ 在参与细胞氧化还原稳态的上皮间充质通讯中的作用。
更新日期:2020-04-24
中文翻译:

成纤维细胞 PPARβ/δ 的缺乏会减少小鼠的非黑色素瘤皮肤癌。
非黑色素瘤皮肤癌 (NMSC) 的发病率在全球范围内呈上升趋势。大多数研究都强调了癌症相关成纤维细胞 (CAF) 在 NMSC 进展中的重要性。然而,对正常成纤维细胞和上皮细胞之间的交流知之甚少。这种通讯的中断会影响肿瘤的发生和肿瘤出现的潜伏期。描述在 NMSC 中介导这种上皮间充质通讯的机制可以确定更有效的靶向治疗。成纤维细胞中的核受体 PPARβ/δ 已被证明可调节邻近的上皮细胞行为,然而,其在皮肤肿瘤发生中的作用仍然未知。使用化学诱导的皮肤癌发生,我们发现 FSPCre-Pparb/dex4 小鼠,其 Pparb/d 基因在成纤维细胞中被选择性删除,与对照小鼠(Pparb / dfl / fl)相比,出现延迟并减少了肿瘤负荷。然而,FSPCre-Pparb/dex4 衍生的肿瘤显示增殖增加,分化没有差异,表明肿瘤起始延迟。网络分析揭示了真皮 Pparb/d 和 TGF-β1 与表皮 NRF2 和 Nox4 之间的联系。体外研究表明,成纤维细胞中 PPARβ/δ 缺乏会增加表皮 Nox4 衍生的 H2O2 产生,从而引发 NRF2 介导的抗氧化反应。我们进一步表明 H2O2 通过 B-Raf-MEK1/2 通路上调 NRF2 mRNA。增强的 NRF2 反应改变了 PTEN、Src 和 AKT 的活性。在体内,我们检测到 B-Raf、MEK1/2、PTEN、Src、与 Pparb/dfl/fl 小鼠相比,在 Pparb/dfl/fl 中首次出现肿瘤之前,FSPCre-Pparb/dex4 小鼠的载体处理和化学处理的表皮中的 AKT 和 AKT。我们的研究揭示了成纤维细胞 PPARβ/δ 在参与细胞氧化还原稳态的上皮间充质通讯中的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号