当前位置:
X-MOL 学术
›
Nat. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective photoinduced cyclodimerization of a prochiral anthracene derivative adsorbed on helical metal nanostructures.
Nature Chemistry ( IF 19.2 ) Pub Date : 2020-04-20 , DOI: 10.1038/s41557-020-0453-0 Xueqin Wei 1, 2 , Junjun Liu 3, 4 , Guang-Jie Xia 5 , Junhong Deng 6 , Peng Sun 3, 6 , Jason J Chruma 1 , Wanhua Wu 1 , Cheng Yang 1 , Yang-Gang Wang 5 , Zhifeng Huang 3, 4
Nature Chemistry ( IF 19.2 ) Pub Date : 2020-04-20 , DOI: 10.1038/s41557-020-0453-0 Xueqin Wei 1, 2 , Junjun Liu 3, 4 , Guang-Jie Xia 5 , Junhong Deng 6 , Peng Sun 3, 6 , Jason J Chruma 1 , Wanhua Wu 1 , Cheng Yang 1 , Yang-Gang Wang 5 , Zhifeng Huang 3, 4
Affiliation
|
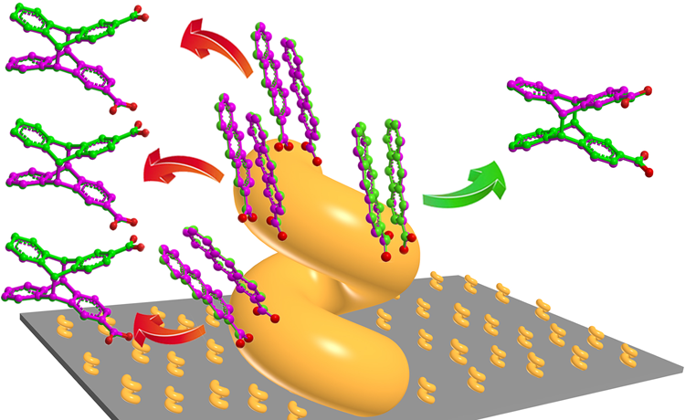
The generation of molecular chirality in the absence of any molecular chiral inductor is challenging and of fundamental interest for developing a better understanding of homochirality. Here, we show the manipulation of molecular chirality through control of the handedness of helical metal nanostructures (referred to as nanohelices) that are produced by glancing angle deposition onto a substrate that rotates in either a clockwise or counterclockwise direction. A prochiral molecule, 2-anthracenecarboxylic acid, is stereoselectively adsorbed on the metal nanohelices as enantiomorphous anti-head-to-head dimers. The dimers show either Si-Si or Re-Re facial stacking depending on the handedness of the nanohelices, which results in a specific enantiopreference during their photoinduced cyclodimerization: a left-handed nanohelix leads to the formation of (+)-cyclodimers, whereas a right-handed one gives (-)-cyclodimers. Density functional theory calculations, in good agreement with the experimental results, point to the enantioselectivity mainly arising from the selective spatial matching of either Si-Si or Re-Re facial stacking at the helical surface; it may also be influenced by chiroplasmonic effects.
中文翻译:

对映选择性光诱导环二聚体的手性蒽衍生物吸附在螺旋金属纳米结构上。
在没有任何分子手性诱导剂的情况下,分子手性的产生是具有挑战性的,并且对于发展对同手性的更好理解具有根本意义。在这里,我们显示了通过控制螺旋金属纳米结构(称为纳米螺旋)的旋向性来控制分子手性,螺旋形金属纳米结构是通过掠过在顺时针或逆时针方向旋转的基板上的掠角沉积而产生的。前手性分子2-蒽羧酸作为对映体反头对头二聚体立体选择性地吸附在金属纳米螺旋上。二聚体根据纳米螺旋的惯用性显示出Si-Si或Re-Re面堆积,这导致在其光诱导的环二聚过程中出现特定的对映体偏好:左手的纳米螺旋导致形成(+)-环二聚体,而右手的纳米螺旋产生(-)-环二聚体。密度泛函理论计算结果与实验结果吻合,指出对映选择性主要源于螺旋表面上Si-Si或Re-Re面堆叠的选择性空间匹配;它也可能受到手性等离子体作用的影响。
更新日期:2020-04-24
中文翻译:

对映选择性光诱导环二聚体的手性蒽衍生物吸附在螺旋金属纳米结构上。
在没有任何分子手性诱导剂的情况下,分子手性的产生是具有挑战性的,并且对于发展对同手性的更好理解具有根本意义。在这里,我们显示了通过控制螺旋金属纳米结构(称为纳米螺旋)的旋向性来控制分子手性,螺旋形金属纳米结构是通过掠过在顺时针或逆时针方向旋转的基板上的掠角沉积而产生的。前手性分子2-蒽羧酸作为对映体反头对头二聚体立体选择性地吸附在金属纳米螺旋上。二聚体根据纳米螺旋的惯用性显示出Si-Si或Re-Re面堆积,这导致在其光诱导的环二聚过程中出现特定的对映体偏好:左手的纳米螺旋导致形成(+)-环二聚体,而右手的纳米螺旋产生(-)-环二聚体。密度泛函理论计算结果与实验结果吻合,指出对映选择性主要源于螺旋表面上Si-Si或Re-Re面堆叠的选择性空间匹配;它也可能受到手性等离子体作用的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号