当前位置:
X-MOL 学术
›
Free Radical Bio. Med.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeting DUSP7 signaling alleviates hepatic steatosis, inflammation and oxidative stress in high fat diet (HFD)-fed mice via suppression of TAK1.
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-04-18 , DOI: 10.1016/j.freeradbiomed.2020.04.009 Liping Wu 1 , Yongcun Liu 2 , Yuan Zhao 3 , Meng Li 1 , Ling Guo 2
Free Radical Biology and Medicine ( IF 7.1 ) Pub Date : 2020-04-18 , DOI: 10.1016/j.freeradbiomed.2020.04.009 Liping Wu 1 , Yongcun Liu 2 , Yuan Zhao 3 , Meng Li 1 , Ling Guo 2
Affiliation
|
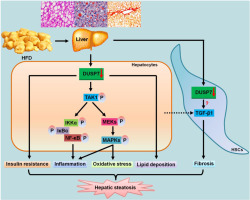
The non-alcoholic fatty liver disease (NAFLD), as a critical liver disease, is still lack of effective treatments because the molecular mechanism revealing the NAFLD pathogenesis remains unclear. Dual specific phosphatase 6 (DUSP7) shows effects on inflammatory response and is a negative feedback mechanism of the mitogen-activated protein kinase (MAPK) superfamily, which are critical factors in regulating NAFLD progression. However, the effects of DUSP7 on hepatic steatosis are still not fully understood. Here, we found that DUSP7 functioned as a negative regulator of NAFLD and in various metabolic disorders. DUSP7 expression was markedly reduced in liver samples from patients with simple hepatic steatosis or non-alcoholic steatohepatitis (NASH), as well as in liver tissues from high fat diet (HFD)-challenged mice or genetically obese (ob/ob) mice. DUSP7 knockout markedly accelerated insulin resistance, glucose intolerance, liver dysfunction, fibrosis and hepatic steatosis in HFD-fed mice. In addition, inflammatory response was significantly exacerbated in HFD-challenged mice with DUSP7 deletion, which was associated with the elevated activation of nuclear factor-κB (NF-κB) and MAPKs signaling pathways. Moreover, oxidative stress was detected in liver of HFD-induced mice, and this phenomenon was aggravated in mice with DUSP7 knockout. Importantly, we demonstrated that DUSP7 physically interacted with transforming growth factor β (TGF-β)-activated kinase (TAK1). DUSP7 deletion considerably promoted the activation of TAK1 in mice after HFD feeding, contributing to the lipid deposition, inflammatory response and reactive oxygen species (ROS) production. Taken together, DUSP7 might function as a protective factor against NAFLD development and metabolic disorder through alleviating dyslipidemia, inflammation and oxidative stress by directly interacting with TAK1 in hepatocytes, which was involved in the suppression of fibrosis. Thus, we may provide an effective strategy for the treatment of hepatic steatosis via targeting DUSP7.
中文翻译:

靶向DUSP7信号传导可通过抑制TAK1减轻高脂饮食(HFD)喂养的小鼠的肝脏脂肪变性,炎症和氧化应激。
非酒精性脂肪性肝病(NAFLD)作为一种严重的肝病,仍缺乏有效的治疗方法,因为揭示NAFLD发病机理的分子机制仍不清楚。双重特异性磷酸酶6(DUSP7)显示出对炎症反应的影响,并且是有丝分裂原激活的蛋白激酶(MAPK)超家族的负反馈机制,这是调节NAFLD进程的关键因素。但是,DUSP7对肝脂肪变性的作用仍未完全了解。在这里,我们发现DUSP7可以作为NAFLD的负调节剂,并在各种代谢性疾病中起作用。在患有单纯性肝脂肪变性或非酒精性脂肪性肝炎(NASH)的患者的肝样品中,以及在高脂饮食(HFD)攻击的小鼠或遗传性肥胖(ob / ob)小鼠的肝组织中,DUSP7的表达均明显降低。敲除DUSP7可以显着加速HFD喂养小鼠的胰岛素抵抗,葡萄糖耐受不良,肝功能障碍,纤维化和肝脂肪变性。此外,在具有DUSP7缺失的HFD攻击小鼠中,炎症反应显着加剧,这与核因子-κB(NF-κB)和MAPKs信号通路的活化增强有关。此外,在HFD诱导的小鼠肝脏中检测到氧化应激,而在敲除DUSP7的小鼠中这种现象加剧了。重要的是,我们证明了DUSP7与转化生长因子β(TGF-β)激活的激酶(TAK1)发生了物理相互作用。喂食HFD后,DUSP7的缺失极大地促进了TAK1的激活,从而促进了脂质沉积,炎症反应和活性氧(ROS)的产生。在一起 DUSP7可能通过与肝细胞中的TAK1直接相互作用来减轻血脂异常,炎症和氧化应激,从而作为抵抗NAFLD发育和代谢紊乱的保护因子,这与抑制纤维化有关。因此,我们可以通过靶向DUSP7提供治疗肝脂肪变性的有效策略。
更新日期:2020-04-20
中文翻译:

靶向DUSP7信号传导可通过抑制TAK1减轻高脂饮食(HFD)喂养的小鼠的肝脏脂肪变性,炎症和氧化应激。
非酒精性脂肪性肝病(NAFLD)作为一种严重的肝病,仍缺乏有效的治疗方法,因为揭示NAFLD发病机理的分子机制仍不清楚。双重特异性磷酸酶6(DUSP7)显示出对炎症反应的影响,并且是有丝分裂原激活的蛋白激酶(MAPK)超家族的负反馈机制,这是调节NAFLD进程的关键因素。但是,DUSP7对肝脂肪变性的作用仍未完全了解。在这里,我们发现DUSP7可以作为NAFLD的负调节剂,并在各种代谢性疾病中起作用。在患有单纯性肝脂肪变性或非酒精性脂肪性肝炎(NASH)的患者的肝样品中,以及在高脂饮食(HFD)攻击的小鼠或遗传性肥胖(ob / ob)小鼠的肝组织中,DUSP7的表达均明显降低。敲除DUSP7可以显着加速HFD喂养小鼠的胰岛素抵抗,葡萄糖耐受不良,肝功能障碍,纤维化和肝脂肪变性。此外,在具有DUSP7缺失的HFD攻击小鼠中,炎症反应显着加剧,这与核因子-κB(NF-κB)和MAPKs信号通路的活化增强有关。此外,在HFD诱导的小鼠肝脏中检测到氧化应激,而在敲除DUSP7的小鼠中这种现象加剧了。重要的是,我们证明了DUSP7与转化生长因子β(TGF-β)激活的激酶(TAK1)发生了物理相互作用。喂食HFD后,DUSP7的缺失极大地促进了TAK1的激活,从而促进了脂质沉积,炎症反应和活性氧(ROS)的产生。在一起 DUSP7可能通过与肝细胞中的TAK1直接相互作用来减轻血脂异常,炎症和氧化应激,从而作为抵抗NAFLD发育和代谢紊乱的保护因子,这与抑制纤维化有关。因此,我们可以通过靶向DUSP7提供治疗肝脂肪变性的有效策略。











































 京公网安备 11010802027423号
京公网安备 11010802027423号