Computational and Theoretical Chemistry ( IF 3.0 ) Pub Date : 2020-04-18 , DOI: 10.1016/j.comptc.2020.112827 N. Turaeva
|
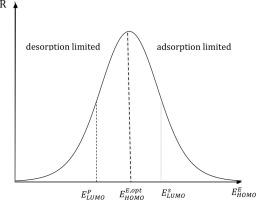
Electron transfers between active sites of the enzyme and the substrate (product) have been taken into account in kinetic equations for multistep enzymatic reactions with some reversible steps by introducing weak and strong chemisorption states for the intermediates based on Wolkenstein‘s electronic theory of catalysis originally developed for semiconductors. As a result, the expression for the reaction rate as a function of energy difference between the highest occupied molecular orbital (HOMO) of the active site of the enzyme and the bonding state of the substrate (product) adsorbed onto the surface of the enzyme has been obtained. The case of interaction between the LUMO of the enzyme and the HOMO of the substrate, as well as different factors impacting on the rate of enzymatic reactions have been also discussed.
中文翻译:

酶促反应的简单电子模型
酶的活性位点与底物(产物)之间的电子转移已在动力学方程中考虑到了多步酶促反应的动力学方程,该过程具有一些可逆的步骤,这是基于最初为Wolkenstein电子催化理论引入的中间体弱和强化学吸附态引起的。半导体。结果,反应速率的表达根据酶活性位点的最高占据分子轨道(HOMO)与吸附在酶表面上的底物(产物)的键合状态之间的能量差而变化。已获得。还讨论了酶的LUMO和底物的HOMO之间相互作用的情况,以及影响酶促反应速率的不同因素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号